Progestin-only pill use over 6 months postpartum
- PMID: 32544400
- PMCID: PMC7572571
- DOI: 10.1016/j.contraception.2020.06.004
Progestin-only pill use over 6 months postpartum
Abstract
Objectives: To determine progestin-only pill (POP) use at 3 and 6 months postpartum among women who chose POPs at the postpartum visit.
Study design: Secondary data analysis of a prospective observational study with telephone interviews at 3 and 6 months postpartum to assess contraceptive use.
Results: Of 440 women who attended the postpartum visit, 92 (20.9%) chose POPs. Current POP use was 44/84 (52.4%) at 3 months, 33/76 (43.4%) at 6 months, and 32/76 (42.1%) at both 3- and 6-month follow-up assessments.
Conclusion: About half of women who plan POP use at the postpartum visit are not using this method at 3 months after delivery.
Implications: About half of women with a prescription for progestin-only pills will be not using this method at 3 months postpartum; further understanding of continued sexual activity and breastfeeding may clarify pregnancy risk for those not reporting modern contraception use during the postpartum period.
Keywords: Breastfeeding; Hormonal contraception; Postpartum contraception; Progestin-only pills.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
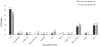
Similar articles
-
Progestin-only contraception prevents bone loss in postpartum breastfeeding women.Contraception. 2012 Apr;85(4):374-80. doi: 10.1016/j.contraception.2011.08.015. Epub 2011 Oct 28. Contraception. 2012. PMID: 22036473
-
New IPPF statement on breastfeeding, fertility and post-partum contraception.IPPF Med Bull. 1990 Apr;24(2):2-4. IPPF Med Bull. 1990. PMID: 12316285
-
Progestin-only pill use and pill switching during breastfeeding.Contraception. 1995 May;51(5):279-81. doi: 10.1016/0010-7824(95)00076-m. Contraception. 1995. PMID: 7628200
-
Hormonal contraception and lactation.J Hum Lact. 1996 Dec;12(4):315-8. doi: 10.1177/089033449601200419. J Hum Lact. 1996. PMID: 9025449 Review.
-
Post-partum contraception.Baillieres Clin Obstet Gynaecol. 1996 Apr;10(1):25-41. doi: 10.1016/s0950-3552(96)80060-5. Baillieres Clin Obstet Gynaecol. 1996. PMID: 8736720 Review.
Cited by
-
Postpartum Hormonal Contraceptive Use and Risk of Depression.JAMA Netw Open. 2025 Mar 3;8(3):e252474. doi: 10.1001/jamanetworkopen.2025.2474. JAMA Netw Open. 2025. PMID: 40163119 Free PMC article.

