COVID-19 cytokine storm: The anger of inflammation
- PMID: 32544563
- PMCID: PMC7260598
- DOI: 10.1016/j.cyto.2020.155151
COVID-19 cytokine storm: The anger of inflammation
Abstract
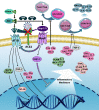
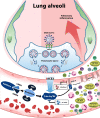
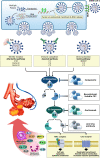
Patients with COVID-19 who require ICU admission might have the cytokine storm. It is a state of out-of-control release of a variety of inflammatory cytokines. The molecular mechanism of the cytokine storm has not been explored extensively yet. The attachment of SARS-CoV-2 spike glycoprotein with angiotensin-converting enzyme 2 (ACE2), as its cellular receptor, triggers complex molecular events that leads to hyperinflammation. Four molecular axes that may be involved in SARS-CoV-2 driven inflammatory cytokine overproduction are addressed in this work. The virus-mediated down-regulation of ACE2 causes a burst of inflammatory cytokine release through dysregulation of the renin-angiotensin-aldosterone system (ACE/angiotensin II/AT1R axis), attenuation of Mas receptor (ACE2/MasR axis), increased activation of [des-Arg9]-bradykinin (ACE2/bradykinin B1R/DABK axis), and activation of the complement system including C5a and C5b-9 components. The molecular clarification of these axes will elucidate an array of therapeutic strategies to confront the cytokine storm in order to prevent and treat COVID-19 associated acute respiratory distress syndrome.
Keywords: ACE2; COVID-19; Cytokine storm; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: An award was given to Iraj Nabipour by “Novo Nordisk Pars” (The Best Innovator of Diabetes in Iran 2013). For the remaining authors none were declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

