ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection
- PMID: 32544566
- PMCID: PMC7293510
- DOI: 10.1016/j.jtos.2020.06.007
ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection
Abstract
Purpose: Conjunctival signs and symptoms are observed in a subset of patients with COVID-19, and SARS-CoV-2 has been detected in tears, raising concerns regarding the eye both as a portal of entry and carrier of the virus. The purpose of this study was to determine whether ocular surface cells possess the key factors required for cellular susceptibility to SARS-CoV-2 entry/infection.
Methods: We analyzed human post-mortem eyes as well as surgical specimens for the expression of ACE2 (the receptor for SARS-CoV-2) and TMPRSS2, a cell surface-associated protease that facilitates viral entry following binding of the viral spike protein to ACE2.
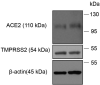
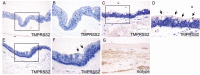
Results: Across all eye specimens, immunohistochemical analysis revealed expression of ACE2 in the conjunctiva, limbus, and cornea, with especially prominent staining in the superficial conjunctival and corneal epithelial surface. Surgical conjunctival specimens also showed expression of ACE2 in the conjunctival epithelium, especially prominent in the superficial epithelium, as well as weak or focal expression in the substantia propria. All eye and conjunctival specimens also expressed TMPRSS2. Finally, Western blot analysis of protein lysates from human corneal epithelium obtained during refractive surgery confirmed expression of ACE2 and TMPRSS2.
Conclusions: Together, these results suggest that ocular surface cells including conjunctiva are susceptible to infection by SARS-CoV-2, and could therefore serve as a portal of entry as well as a reservoir for person-to-person transmission of this virus. This highlights the importance of safety practices including face masks and ocular contact precautions in preventing the spread of COVID-19 disease.
Keywords: ACE2; COVID-19; Conjunctiva; Cornea; Human; Ocular surface; SARS-CoV-2.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Update of
-
ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection.bioRxiv [Preprint]. 2020 May 9:2020.05.09.086165. doi: 10.1101/2020.05.09.086165. bioRxiv. 2020. Update in: Ocul Surf. 2020 Oct;18(4):537-544. doi: 10.1016/j.jtos.2020.06.007. PMID: 32511393 Free PMC article. Updated. Preprint.
Comment in
-
SARS-CoV-2 receptor ACE2 is expressed in human conjunctival tissue, especially in diseased conjunctival tissue.Ocul Surf. 2021 Jan;19:249-251. doi: 10.1016/j.jtos.2020.09.010. Epub 2020 Sep 28. Ocul Surf. 2021. PMID: 32991984 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

