Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer
- PMID: 32545200
- PMCID: PMC7352546
- DOI: 10.3390/cancers12061531
Mass Spectrometric Comparison of HPV-Positive and HPV-Negative Oropharyngeal Cancer
Abstract
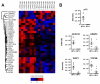
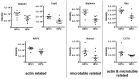
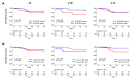
Squamous cell carcinoma of the head and neck (HNSCC) consist of two distinct biological entities. While the numbers of classical, tobacco-induced HNSCC are declining, tumors caused by human papillomavirus (HPV) infection are increasing in many countries. HPV-positive HNSCC mostly arise in the oropharynx and are characterized by an enhanced sensitivity towards radiotherapy and a favorable prognosis. To identify molecular differences between both entities on the protein level, we conducted a mass spectrometric comparison of eight HPV-positive and nine HPV-negative oropharyngeal tumors (OPSCC). Overall, we identified 2051 proteins, of which 31 were found to be differentially expressed. Seventeen of these can be assorted to three functional groups, namely DNA replication, nuclear architecture and cytoskeleton regulation, with the differences in the last group potentially reflecting an enhanced migratory and invasive capacity. Furthermore, a number of identified proteins have been described to directly impact on DNA double-strand break repair or radiation sensitivity (e.g., SLC3A2, cortactin, RBBP4, Numa1), offering explanations for the differential prognosis. The unequal expression of three proteins (SLC3A2, MCM2 and lamin B1) was confirmed by immunohistochemical staining using a tissue microarray containing 205 OPSCC samples. The expression levels of SLC3A2 and lamin B1 were found be of prognostic relevance in patients with HPV-positive and HPV-negative OPSCC, respectively.
Keywords: HPV; head and neck cancer; mass spectrometry; oropharynx.
Conflict of interest statement
The authors declare no confilict of interest.
Figures




References
-
- Conway D.I., Brenner D.R., McMahon A.D., Macpherson L.M., Agudo A., Ahrens W., Bosetti C., Brenner H., Castellsague X., Chen C., et al. Estimating and explaining the effect of education and income on head and neck cancer risk: INHANCE consortium pooled analysis of 31 case-control studies from 27 countries. Int. J. Cancer. 2015;136:1125–1139. doi: 10.1002/ijc.29063. - DOI - PMC - PubMed
-
- Chaturvedi A.K., Engels E.A., Pfeiffer R.M., Hernandez B.Y., Xiao W., Kim E., Jiang B., Goodman M.T., Sibug-Saber M., Cozen W., et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. - DOI - PMC - PubMed
-
- Mehanna H., Beech T., Nicholson T., El-Hariry I., McConkey C., Paleri V., Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

