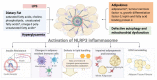
NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases
- PMID: 32545355
- PMCID: PMC7312293
- DOI: 10.3390/ijms21114184
NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases
Abstract
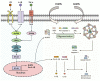
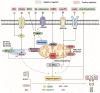
Adipose tissue is an active endocrine and immune organ that controls systemic immunometabolism via multiple pathways. Diverse immune cell populations reside in adipose tissue, and their composition and immune responses vary with nutritional and environmental conditions. Adipose tissue dysfunction, characterized by sterile low-grade chronic inflammation and excessive immune cell infiltration, is a hallmark of obesity, as well as an important link to cardiometabolic diseases. Amongst the pro-inflammatory factors secreted by the dysfunctional adipose tissue, interleukin (IL)-1β, induced by the NLR family pyrin domain-containing 3 (NLRP3) inflammasome, not only impairs peripheral insulin sensitivity, but it also interferes with the endocrine and immune functions of adipose tissue in a paracrine manner. Human studies indicated that NLRP3 activity in adipose tissues positively correlates with obesity and its metabolic complications, and treatment with the IL-1β antibody improves glycaemia control in type 2 diabetic patients. In mouse models, genetic or pharmacological inhibition of NLRP3 activation pathways or IL-1β prevents adipose tissue dysfunction, including inflammation, fibrosis, defective lipid handling and adipogenesis, which in turn alleviates obesity and its related metabolic disorders. In this review, we summarize both the negative and positive regulators of NLRP3 inflammasome activation, and its pathophysiological consequences on immunometabolism. We also discuss the potential therapeutic approaches to targeting adipose tissue inflammasome for the treatment of obesity and its related metabolic disorders.
Keywords: IL-1β; NLRP3 inflammasome; adipose tissue; immunometabolism; insulin sensitivity; metabolic disease; obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

