Apelin Controls Angiogenesis-Dependent Glioblastoma Growth
- PMID: 32545380
- PMCID: PMC7312290
- DOI: 10.3390/ijms21114179
Apelin Controls Angiogenesis-Dependent Glioblastoma Growth
Abstract
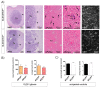
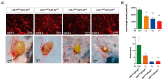
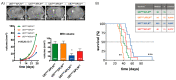
Glioblastoma (GBM) present with an abundant and aberrant tumor neo-vasculature. While rapid growth of solid tumors depends on the initiation of tumor angiogenesis, GBM also progress by infiltrative growth and vascular co-option. The angiogenic factor apelin (APLN) and its receptor (APLNR) are upregulated in GBM patient samples as compared to normal brain tissue. Here, we studied the role of apelin/APLNR signaling in GBM angiogenesis and growth. By functional analysis of apelin in orthotopic GBM mouse models, we found that apelin/APLNR signaling is required for in vivo tumor angiogenesis. Knockdown of tumor cell-derived APLN massively reduced the tumor vasculature. Additional loss of the apelin signal in endothelial tip cells using the APLN-knockout (KO) mouse led to a further reduction of GBM angiogenesis. Direct infusion of the bioactive peptide apelin-13 rescued the vascular loss-of-function phenotype specifically. In addition, APLN depletion massively reduced angiogenesis-dependent tumor growth. Consequently, survival of GBM-bearing mice was significantly increased when APLN expression was missing in the brain tumor microenvironment. Thus, we suggest that targeting vascular apelin may serve as an alternative strategy for anti-angiogenesis in GBM.
Keywords: APLN; APLNR; Apelin-13; GBM angiogenesis; glioblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kleihues P., Cavenee W.K. Pathology and Genetics of Tumours of the Nervous System. IARC Press; Lyon, France: 2000.
-
- Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. - DOI - PubMed
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W., et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

