Exploring the Microalga Euglena cantabrica by Pressurized Liquid Extraction to Obtain Bioactive Compounds
- PMID: 32545497
- PMCID: PMC7345716
- DOI: 10.3390/md18060308
Exploring the Microalga Euglena cantabrica by Pressurized Liquid Extraction to Obtain Bioactive Compounds
Abstract
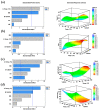
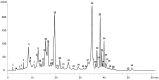
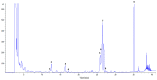
In the present study, the chemical composition of the microalga Euglena cantabrica was investigated. The extraction of bioactive compounds was done using pressurized liquid extraction (PLE) at different temperatures (40-180 °C) and using green solvents (ethanol-water mixtures). A statistical design of experiments was used to optimize the maximum antioxidant capacity of the extracts by response surface methodology. The antioxidant capacity was determined through the inhibition of 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals, while the chemical analyses of the extracts were carried out using different chromatographic techniques. Chlorophylls and carotenoids were analyzed by high-performance liquid chromatography coupled to a diode array detector and mass spectrometry (HPLC-DAD-MS/MS) and carbohydrates by gas chromatography with flame ionization detection (GC-FID) and high-pressure size-exclusion chromatography coupled to an evaporative light-scattering detector (HPSEC-ELSD). The results showed different possibilities for the extraction conditions, depending on the desired bioactivity or chemical composition. Briefly, (i) mixtures of ethanol-water containing around 40% ethanol at 180 °C gave the best antioxidant capacity, (ii) mixtures containing around 50% ethanol at 110 °C gave the best yield of β-glucan paramylon, and (iii) the use of pure ethanol at a low temperature (40 °C) is the best choice for the recovery of carotenoids such as diatoxanthin. Summing up, E. cantabrica seems to be a good candidate to be used in biorefinery to obtain different bioactive compounds.
Keywords: Euglena cantabrica; carbohydrates; carotenoid; microalga; paramylon; pressurized liquid extraction; response surface.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum.Food Res Int. 2017 Sep;99(Pt 3):1056-1065. doi: 10.1016/j.foodres.2016.04.022. Epub 2016 Apr 23. Food Res Int. 2017. PMID: 28865617
-
Development of green extraction processes for Nannochloropsis gaditana biomass valorization.Electrophoresis. 2018 Apr 23. doi: 10.1002/elps.201800122. Online ahead of print. Electrophoresis. 2018. PMID: 29683520
-
Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD.J Sep Sci. 2005 Nov;28(16):2111-9. doi: 10.1002/jssc.200500185. J Sep Sci. 2005. PMID: 16318207
-
Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds.J Chromatogr A. 2013 Oct 25;1313:78-95. doi: 10.1016/j.chroma.2013.07.051. Epub 2013 Jul 16. J Chromatogr A. 2013. PMID: 23899380 Review.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Enhancing the Biological Effects of Bioactive Compounds from Microalgae through Advanced Processing Techniques: Pioneering Ingredients for Next-Generation Food Production.Foods. 2024 Jun 8;13(12):1811. doi: 10.3390/foods13121811. Foods. 2024. PMID: 38928753 Free PMC article. Review.
-
An Integrated Approach for the Valorization of Sea Bass (Dicentrarchus labrax) Side Streams: Evaluation of Contaminants and Development of Antioxidant Protein Extracts by Pressurized Liquid Extraction.Foods. 2021 Mar 6;10(3):546. doi: 10.3390/foods10030546. Foods. 2021. PMID: 33800768 Free PMC article.
-
A critical look at challenges and future scopes of bioactive compounds and their incorporations in the food, energy, and pharmaceutical sector.Environ Sci Pollut Res Int. 2022 May;29(24):35518-35541. doi: 10.1007/s11356-022-19423-4. Epub 2022 Mar 2. Environ Sci Pollut Res Int. 2022. PMID: 35233673 Free PMC article. Review.
References
-
- Allen E.J., Allen E.J., Nelson E.W. On the artificial culture of marine plankton organisms. J. Mar. Biol. Assoc. U.K. 1910;8:421–474. doi: 10.1017/S0025315400073690. - DOI
-
- Herrero M., Sánchez-Camargo A.P., Cifuentes A., Ibáñez E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TRAC-Trends Anal. Chem. 2015;71:26–38. doi: 10.1016/j.trac.2015.01.018. - DOI
-
- Mobin S., Alam F. Some Promising Microalgal Species for Commercial Applications: A review. Energy Procedia. 2017;110:510–517. doi: 10.1016/j.egypro.2017.03.177. - DOI
-
- Kottuparambil S., Thankamony R.L., Agusti S. Euglena as a potential natural source of value-added metabolites. A review. Algal Res. 2019;37:154–159. doi: 10.1016/j.algal.2018.11.024. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

