Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays
- PMID: 32545533
- PMCID: PMC7352801
- DOI: 10.3390/ijms21124197
Recombinant Adeno-Associated Viral Vectors (rAAV)-Vector Elements in Ocular Gene Therapy Clinical Trials and Transgene Expression and Bioactivity Assays
Abstract
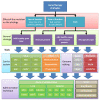
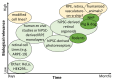
Inherited retinal dystrophies and optic neuropathies cause chronic disabling loss of visual function. The development of recombinant adeno-associated viral vectors (rAAV) gene therapies in all disease fields have been promising, but the translation to the clinic has been slow. The safety and efficacy profiles of rAAV are linked to the dose of applied vectors. DNA changes in the rAAV gene cassette affect potency, the expression pattern (cell-specificity), and the production yield. Here, we present a library of rAAV vectors and elements that provide a workflow to design novel vectors. We first performed a meta-analysis on recombinant rAAV elements in clinical trials (2007-2020) for ocular gene therapies. We analyzed 33 unique rAAV gene cassettes used in 57 ocular clinical trials. The rAAV gene therapy vectors used six unique capsid variants, 16 different promoters, and six unique polyadenylation sequences. Further, we compiled a list of promoters, enhancers, and other sequences used in current rAAV gene cassettes in preclinical studies. Then, we give an update on pro-viral plasmid backbones used to produce the gene therapy vectors, inverted terminal repeats, production yield, and rAAV safety considerations. Finally, we assess rAAV transgene and bioactivity assays applied to cells or organoids in vitro, explants ex vivo, and clinical studies.
Keywords: Cas9; Keywords. retina; adeno-associated virus (AAV); biological activity assay (BAA); enhancer; polyadenylation; pro-viral plasmid; promoter; retinal pigment epithelium (RPE); transgene expression assay (TEA).
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationship that could be construed as a potential conflict of interest. The LUMC is owner of a pending patent on the use of CRB2 gene therapy vectors (PCT/NL2014/050549) and receives license income and research funds from HORAMA. JW is a full-term employee of LUMC and acts for LUMC as a temporary consultant for HORAMA. JW is mentioned as an inventor on the patent application. TMB declares no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

