Transcriptomically Revealed Oligo-Fucoidan Enhances the Immune System and Protects Hepatocytes via the ASGPR/STAT3/HNF4A Axis
- PMID: 32545625
- PMCID: PMC7355575
- DOI: 10.3390/biom10060898
Transcriptomically Revealed Oligo-Fucoidan Enhances the Immune System and Protects Hepatocytes via the ASGPR/STAT3/HNF4A Axis
Abstract
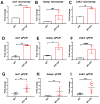
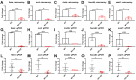
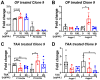
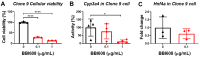
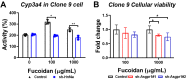
Oligo-fucoidan, a sulfated polysaccharide extracted from brown seaweed, exhibits anti-inflammatory and anti-tumor effects. However, the knowledge concerning the detailed mechanism of oligo-fucoidan on liver cells is obscure. In this study, we investigate the effect of oligo-fucoidan in normal hepatocytes by transcriptomic analysis. Using an oligo-fucoidan oral gavage in wild-type adult zebrafish, we find that oligo-fucoidan pretreatment enhances the immune system and anti-viral genes in hepatocytes. Oligo-fucoidan pretreatment also decreases the expression of lipogenic enzymes and liver fibrosis genes. Using pathway analysis, we identify hepatocyte nuclear factor 4 alpha (HNF4A) to be the potential driver gene. We further investigate whether hepatocyte nuclear factor 4 alpha (HNF4A) could be induced by oligo-fucoidan and the underlying mechanism. Therefore, a normal hepatocyte clone 9 cell as an in vitro model was used. We demonstrate that oligo-fucoidan increases cell viability, Cyp3a4 activity, and Hnf4a expression in clone 9 cells. We further demonstrate that oligo-fucoidan might bind to asialoglycoprotein receptors (ASGPR) in normal hepatocytes through both in vitro and in vivo competition assays. This binding, consequently activating the signal transducer and activator of transcription 3 (STAT3), increases the expression of the P1 isoform of HNF4A. According to our data, we suggest that oligo-fucoidan not only enhances the gene expression associated with anti-viral ability and immunity, but also increases P1-HNF4A levels through ASGPR/STAT3 axis, resulting in protecting hepatocytes.
Keywords: hepatocyte; hepatocyte nuclear factor 4 alpha (HNF4A); oligo-fucoidan.
Conflict of interest statement
Hi-Q Marine Biotech International Ltd. provided the oligo-fucoidan. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Guo C., Li J., Chen K., Li S., Feng J., Liu T., Wang F., Zhang R., Xu S., Zhou Y., et al. Protective effect of fucoidan from Fucus vesiculosus on liver fibrosis via the TGF-β1/Smad pathway-mediated inhibition of extracellular matrix and autophagy. Drug Des. Dev. Ther. 2016;10:619–630. doi: 10.2147/DDDT.S98740. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

