Asymmetric Synthesis of Tailor-Made Amino Acids Using Chiral Ni(II) Complexes of Schiff Bases. An Update of the Recent Literature
- PMID: 32545684
- PMCID: PMC7356839
- DOI: 10.3390/molecules25122739
Asymmetric Synthesis of Tailor-Made Amino Acids Using Chiral Ni(II) Complexes of Schiff Bases. An Update of the Recent Literature
Abstract
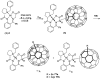
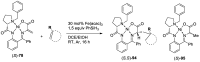
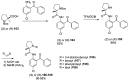
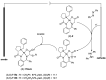
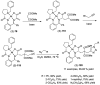
Tailor-made amino acids are indispensable structural components of modern medicinal chemistry and drug design. Consequently, stereo-controlled preparation of amino acids is the area of high research activity. Over last decade, application of Ni(II) complexes of Schiff bases derived from glycine and chiral tridentate ligands has emerged as a leading methodology for the synthesis of various structural types of amino acids. This review article summarizes examples of asymmetric synthesis of tailor-made α-amino acids via the corresponding Ni(II) complexes, reported in the literature over the last four years. A general overview of this methodology is provided, with the emphasis given to practicality, scalability, cost-structure and recyclability of the chiral tridentate ligands.
Keywords: Schiff bases; asymmetric synthesis; chiral tridentate ligands; square-planar Ni(II) complexes; tailor-made amino acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







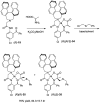


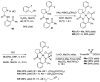




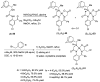
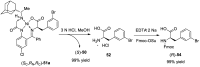
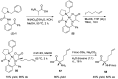

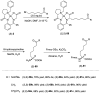

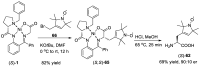


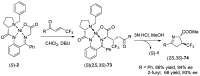

















References
-
- Soloshonok V.A., Cai C., Hruby V.J., Meervelt L.V. Asymmetric Synthesis of Novel Highly Sterically Constrained (2S,3S)-3-Methyl-3-Trifluoromethyl- and (2S,3S,4R)-3-Trifluoromethyl-4-Methylpyroglutamic Acids. Tetrahedron. 1999;55:12045–12058. doi: 10.1016/S0040-4020(99)00710-3. - DOI
-
- Soloshonok V.A., Izawa K. In: Asymmetric Synthesis and Application of α-Amino Acids, ACS Symposium Series #1009. Soloshonok V.A., Izawa K., editors. Oxford University Press; Washington, DC, USA: 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

