Pleiotropic Role of Notch Signaling in Human Skin Diseases
- PMID: 32545758
- PMCID: PMC7353046
- DOI: 10.3390/ijms21124214
Pleiotropic Role of Notch Signaling in Human Skin Diseases
Abstract
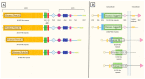
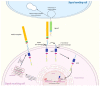
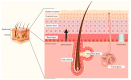
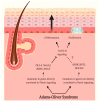
Notch signaling orchestrates the regulation of cell proliferation, differentiation, migration and apoptosis of epidermal cells by strictly interacting with other cellular pathways. Any disruption of Notch signaling, either due to direct mutations or to an aberrant regulation of genes involved in the signaling route, might lead to both hyper- or hypo-activation of Notch signaling molecules and of target genes, ultimately inducing the onset of skin diseases. The mechanisms through which Notch contributes to the pathogenesis of skin diseases are multiple and still not fully understood. So far, Notch signaling alterations have been reported for five human skin diseases, suggesting the involvement of Notch in their pathogenesis: Hidradenitis Suppurativa, Dowling Degos Disease, Adams-Oliver Syndrome, Psoriasis and Atopic Dermatitis. In this review, we aim at describing the role of Notch signaling in the skin, particularly focusing on the principal consequences associated with its alterations in these five human skin diseases, in order to reorganize the current knowledge and to identify potential cellular mechanisms in common between these pathologies.
Keywords: Notch pathway; differentiation; proliferation; skin disorder.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Talora C., Campese A.F., Bellavia D., Felli M.P., Vacca A., Gulino A., Screpanti I. Notch signaling and diseases: An evolutionary journey from a simple beginning to complex outcomes. Biochimica et Biophysica Acta (BBA)-Mol. Basis Dis. 2008;1782:489–497. doi: 10.1016/j.bbadis.2008.06.008. - DOI - PubMed

