Cotargeting of XPO1 Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Acute Myeloid Leukemia
- PMID: 32545904
- PMCID: PMC7352446
- DOI: 10.3390/cancers12061574
Cotargeting of XPO1 Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Acute Myeloid Leukemia
Abstract
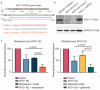
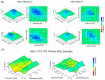
Acute myeloid leukemia (AML) is a hematopoietic stem-cell-derived leukemia with often successive derived driver mutations. Late onset acquisition of internal tandem duplication in FLT3 (FLT3-ITD) at a high variant allele frequency often contributes to full transformation to a highly proliferative, rapidly progressive disease with poor outcome. The FLT3-ITD mutation is targetable with approved FLT3 small molecule inhibitors, including midostaurin and gilteritinib. However, outside of patients receiving allogeneic transplant, most patients fail to respond or relapse, suggesting alternative approaches of therapy will be required. We employed genome-wide pooled CRISPR knockout screening as a method for large-scale identification of targets whose knockout produces a phenotypic effect that enhances the antitumor properties of FLT3 inhibitors. Among the candidate targets we identified the effect of XPO1 knockout to be synergistic with midostaurin treatment. Next, we validated the genetic finding with pharmacologic combination of the slowly reversible XPO1 inhibitor selinexor with midostaurin and gilteritinib in FLT3-ITD AML cell lines and primary patient samples. Lastly, we demonstrated improved survival with either combination therapy compared to its monotherapy components in an aggressive AML murine model, supporting further evaluation and rapid clinical translation of this combination strategy.
Keywords: AML; CRISPR-Cas9 screening; FLT3; XPO1; synergism.
Conflict of interest statement
J.S.B consults for AbbVie, AstraZeneca, and KITE Pharma. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Inhibition of Bcl-2 Synergistically Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Preclinical Models of FLT3-Mutated Acute Myeloid Leukemia.Clin Cancer Res. 2019 Nov 15;25(22):6815-6826. doi: 10.1158/1078-0432.CCR-19-0832. Epub 2019 Jul 18. Clin Cancer Res. 2019. PMID: 31320594 Free PMC article.
-
Gilteritinib: a novel FLT3 inhibitor for acute myeloid leukemia.Biomark Res. 2019 Sep 11;7:19. doi: 10.1186/s40364-019-0170-2. eCollection 2019. Biomark Res. 2019. PMID: 31528345 Free PMC article. Review.
-
Quizartinib, a selective FLT3 inhibitor, maintains antileukemic activity in preclinical models of RAS-mediated midostaurin-resistant acute myeloid leukemia cells.Oncotarget. 2020 Mar 17;11(11):943-955. doi: 10.18632/oncotarget.27489. eCollection 2020 Mar 17. Oncotarget. 2020. PMID: 32215183 Free PMC article.
-
The Multi-Kinase Inhibitor EC-70124 Is a Promising Candidate for the Treatment of FLT3-ITD-Positive Acute Myeloid Leukemia.Cancers (Basel). 2022 Mar 21;14(6):1593. doi: 10.3390/cancers14061593. Cancers (Basel). 2022. PMID: 35326743 Free PMC article.
-
FLT3 Mutations in Acute Myeloid Leukemia: Key Concepts and Emerging Controversies.Front Oncol. 2020 Dec 23;10:612880. doi: 10.3389/fonc.2020.612880. eCollection 2020. Front Oncol. 2020. PMID: 33425766 Free PMC article. Review.
Cited by
-
The prognostic significance of genetics in acute myeloid leukemia under venetoclax-based treatment.Ann Hematol. 2024 Dec;103(12):5019-5033. doi: 10.1007/s00277-024-06050-x. Epub 2024 Oct 29. Ann Hematol. 2024. PMID: 39467855 Review.
-
Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia.J Hematol Oncol. 2020 Oct 19;13(1):139. doi: 10.1186/s13045-020-00973-4. J Hematol Oncol. 2020. PMID: 33076970 Free PMC article.
-
High-Throughput CRISPR Screening in Hematological Neoplasms.Cancers (Basel). 2022 Jul 25;14(15):3612. doi: 10.3390/cancers14153612. Cancers (Basel). 2022. PMID: 35892871 Free PMC article. Review.
-
Targeting OXPHOS de novo purine synthesis as the nexus of FLT3 inhibitor-mediated synergistic antileukemic actions.Sci Adv. 2022 Sep 16;8(37):eabp9005. doi: 10.1126/sciadv.abp9005. Epub 2022 Sep 16. Sci Adv. 2022. PMID: 36112677 Free PMC article.
-
Mechanisms of myeloid leukemogenesis: Current perspectives and therapeutic objectives.Blood Rev. 2023 Jan;57:100996. doi: 10.1016/j.blre.2022.100996. Epub 2022 Aug 2. Blood Rev. 2023. PMID: 35989139 Free PMC article. Review.
References
-
- Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. - DOI - PMC - PubMed
-
- Schlenk R.F., Kayser S., Bullinger L., Kobbe G., Casper J., Ringhoffer M., Held G., Brossart P., Lübbert M., Salih H.R., et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124:3441–3449. doi: 10.1182/blood-2014-05-578070. - DOI - PubMed
-
- Garg M., Nagata Y., Kanojia D., Mayakonda A., Yoshida K., Haridas Keloth S., Zang Z.J., Okuno Y., Shiraishi Y., Chiba K., et al. Profiling of somatic mutations in acute myeloid leukemia with FLT3-ITD at diagnosis and relapse. Blood. 2015;126:2491–2501. doi: 10.1182/blood-2015-05-646240. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

