Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux
- PMID: 32546086
- PMCID: PMC8354598
- DOI: 10.1080/15548627.2020.1782034
Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux
Abstract
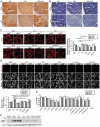
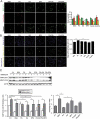
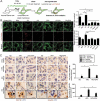
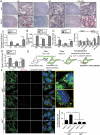
Recently, we identified a novel mechanism of lipotoxicity in the kidney proximal tubular cells (PTECs); lipid overload stimulates macroautophagy/autophagy for the renovation of plasma and organelle membranes to maintain the integrity of the PTECs. However, this autophagic activation places a burden on the lysosomal system, leading to a downstream suppression of autophagy, which manifests as phospholipid accumulation and inadequate acidification in lysosomes. Here, we investigated whether pharmacological correction by eicosapentaenoic acid (EPA) supplementation could restore autophagic flux and alleviate renal lipotoxicity. EPA supplementation to high-fat diet (HFD)-fed mice reduced several hallmarks of lipotoxicity in the PTECs, such as phospholipid accumulation in the lysosome, mitochondrial dysfunction, inflammation, and fibrosis. In addition to improving the metabolic syndrome, EPA alleviated renal lipotoxicity via several mechanisms. EPA supplementation to HFD-fed mice or the isolated PTECs cultured in palmitic acid (PA) restored lysosomal function with significant improvements in the autophagic flux. The PA-induced redistribution of phospholipids from cellular membranes into lysosomes and the HFD-induced accumulation of SQSTM1/p62 (sequestosome 1), an autophagy substrate, during the temporal and genetic ablation of autophagy were significantly reduced by EPA, indicating that EPA attenuated the HFD-mediated increases in autophagy demand. Moreover, a fatty acid pulse-chase assay revealed that EPA promoted lipid droplet (LD) formation and transfer from LDs to the mitochondria for beta-oxidation. Noteworthy, the efficacy of EPA on lipotoxicity is autophagy-dependent and cell-intrinsic. In conclusion, EPA counteracts lipotoxicity in the proximal tubule by alleviating autophagic numbness, making it potentially suitable as a novel treatment for obesity-related kidney diseases.Abbreviations: 4-HNE: 4-hydroxy-2-nonenal; ACTB: actin beta; ADGRE1/F4/80: adhesion G protein-coupled receptor E1; ATG: autophagy-related; ATP: adenosine triphosphate; BODIPY: boron-dipyrromethene; BSA: bovine serum albumin; cKO: conditional knockout; CML: N-carboxymethyllysine; COL1A1: collagen type I alpha 1 chain; COX: cytochrome c oxidase; CTRL: control; DGAT: diacylglycerol O-acyltransferase; EPA: eicosapentaenoic acid; FA: fatty acid; FFA: free fatty acid; GFP: green fluorescent protein; HFD: high-fat diet; iKO: inducible knockout; IRI: ischemia-reperfusion injury; LAMP1: lysosomal-associated membrane protein 1; LD: lipid droplet; LRP2: low density lipoprotein receptor-related protein 2; MAP1LC3: microtubule-associated protein 1 light chain 3; MTORC1: mechanistic target of rapamycin kinase complex 1; OA: oleic acid; PAS: periodic-acid Schiff; PPAR: peroxisome proliferator activated receptor; PPARGC1/PGC1: peroxisome proliferator activated receptor, gamma, coactivator 1; PTEC: proximal tubular epithelial cell; ROS: reactive oxygen species; RPS6: ribosomal protein S6; SDH: succinate dehydrogenase complex; SFC/MS/MS: supercritical fluid chromatography triple quadrupole mass spectrometry; SQSTM1/p62: sequestosome 1; TFEB: transcription factor EB; TG: triglyceride; TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling.
Keywords: Autophagic flux; autophagy; lipid droplet; lysosome; mitochondria.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
