The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection
- PMID: 32546188
- PMCID: PMC7296907
- DOI: 10.1186/s13054-020-03062-7
The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection
Abstract
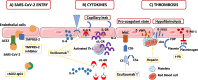
In severe SARS-CoV-2 infections, emerging data including recent histopathological studies have emphasized the crucial role of endothelial cells (ECs) in vascular dysfunction, immunothrombosis, and inflammation.Histopathological studies have evidenced direct viral infection of ECs, endotheliitis with diffuse endothelial inflammation, and micro- and macrovascular thrombosis both in the venous and arterial circulations. Venous thrombotic events, particularly pulmonary embolism, with elevated D-dimer and coagulation activation are highly prevalent in COVID-19 patients. The pro-inflammatory cytokine storm, with elevated levels of interleukin-6 (IL-6), IL-2 receptor, and tumor necrosis factor-α, could also participate in endothelial dysfunction and leukocyte recruitment in the microvasculature. COVID-19-induced endotheliitis may explain the systemic impaired microcirculatory function in different organs in COVID-19 patients. Ongoing trials directly and indirectly target COVID-19-related endothelial dysfunctions: i.e., a virus-cell entry using recombinant angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS-2) blockade, coagulation activation, and immunomodulatory therapies, such as anti-IL-6 strategies. Studies focusing on endothelial dysfunction in COVID-19 patients are warranted as to decipher their precise role in severe SARS-CoV-2 infection and organ dysfunction and to identify targets for further interventions.
Keywords: COVID-19; Cytokines; Endothelial cells; Endothelial dysfunction; SARS-CoV-2; Thrombosis.
Conflict of interest statement
SP received a research grant from the French Intensive Care Society, one from the European Society of Intensive Care Medicine, and one from the Zoll foundation. EA has received fees for lectures from MSD, Pfizer, and Alexion. His institution and research group have received support from Baxter, Jazz Pharma, Fisher&Payckle, Gilead, Alexion, and Ablynx. LZ received a research grant from Jazz Pharma.
Figures
References
-
- Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 [cited 2020 May 5]; Available from: http://doi.wiley.com/10.1002/jmv.25884. - DOI - PMC - PubMed
-
- Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 [cited 2020 May 5]; Available from: https://jamanetwork.com/journals/jama/fullarticle/2765302. - PubMed
-
- Roumenina LT, Rayes J, Frimat M, Fremeaux-Bacchi V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol Rev. 2016;274:307–329. - PubMed
-
- McKenzie JAG, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

