Safety of pharmacologic interventions for neuropsychiatric symptoms in dementia: a systematic review and network meta-analysis
- PMID: 32546202
- PMCID: PMC7298771
- DOI: 10.1186/s12877-020-01607-7
Safety of pharmacologic interventions for neuropsychiatric symptoms in dementia: a systematic review and network meta-analysis
Abstract
Background: Prescribing trends suggest that pharmacologic alternatives to antipsychotics are gaining in popularity, but randomized trial (RCT) data of their comparative safety is scarce. Our objective was to describe the comparative safety of pharmacologic interventions for treating neuropsychiatric symptoms in dementia.
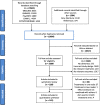
Methods: We searched MEDLINE, EMBASE, CENTRAL, CINAHL, and PsycINFO, from inception to May 28, 2019, for studies of pharmacologic interventions used to treat neuropsychiatric symptoms in dementia. Dementia care partners selected fracture risk as our primary outcome. Pairs of reviewers, working independently, conducted all study screening, data abstraction, and risk of bias appraisal. We conducted Bayesian random-effects network meta-analyses (NMAs) using data from RCTs to derive odds ratios (ORs). In secondary analyses, we conducted frequentist random-effects NMAs using data from RCTs and Bayesian three-level hierarchical random-effects NMAs incorporating data from RCTs and non-randomized studies.
Results: Our systematic review included 209 randomized and non-randomized studies (889,378 persons with dementia). In NMAs of data from randomized trials, there were no increased odds of fracture associated with any intervention in primary analyses; however, data were sparse. We found increased odds of cerebrovascular events associated with antipsychotics (odds ratio [OR] 2.12, 95% credible interval [CrI] 1.29 to 3.62; number needed to harm [NNH] = 99) and increased odds of falls associated with dextromethorphan-quinidine (OR 4.16, 95% CrI 1.47 to 14.22; NNH = 55) compared to placebo in persons with dementia. In a subgroup of persons with Alzheimer disease, antipsychotics were associated with increased odds of fracture compared to anticonvulsants (OR 54.1, 95% CrI 1.15 to 38,300; NNH = 18). In older persons (mean age ≥ 80 years) with dementia, anticonvulsants were associated with increased odds of death compared to placebo (OR 8.36, 95% CrI 1.17 to 203.4; NNH = 35) and antipsychotics were associated with increased odds of death compared to antidepressants (OR 5.28, 95% CrI 1.06 to 3.51; NNH = 47).
Conclusion: Although antipsychotics were associated with greater harm than antidepressants and anticonvulsants in subgroups of persons with dementia, medications used in lieu of antipsychotics for treating neuropsychiatric symptoms in dementia, such as anticonvulsants and dextromethorphan-quinidine, were also associated with harm. Decision-making concerning treatments prescribed in lieu of antipsychotics should include potential harms.
Prospero registration: CRD42017050130.
Keywords: Dementia; Harm; Meta-analysis; Network meta-analysis; Neuropsychiatric symptoms; Systematic review.
Conflict of interest statement
JAW was supported by a Canadian Institutes of Health Research (CIHR) doctoral research award and the University of Toronto Department of Medicine Eliot Phillipson Clinician Scientist Training program during the completion of this manuscript. ACT is on the editorial board for BMC Medicine and funded by a Tier 2 Canada Research Chair in Knowledge Synthesis. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. AAV is funded by a European Union’s Horizon 2020 grant [No. 754936]. All other authors have nothing to disclose.
Figures


References
-
- Patterson C. World Alzheimer Report 2018 - The state of the art of dementia research: New frontiers. London: Alzheimer’s Disease International; 2018.
-
- Buettner L, Ferrario J. Therapeutic recreation-nursing team: a therapeutic intervention for nursing home residents with dementia. Ann Ther Recreation. 1998;7:21–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

