Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms
- PMID: 32546752
- PMCID: PMC7297973
- DOI: 10.1038/s41398-020-00876-5
Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms
Abstract
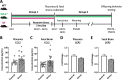
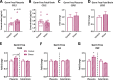
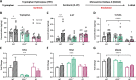
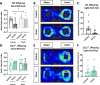
Prenatal stress (PNS) is associated with neuropsychiatric disorders in offspring, including anxiety, depression, and autism spectrum disorders. There is mounting evidence that these behavioral phenotypes have origins in utero. Maternal microbes, inflammation, and serotonergic dysfunction have been implicated as potential mediators of the behavioral consequences of PNS; whether and how these systems interact is unclear. Here, we examine the effects of PNS in utero using late-gestation maternal restraint stress in wild-type (WT), germ-free (GF), and CCL2-/- genetic knock-out (KO) mice. In WT mice, PNS leads to placental and fetal brain inflammation, including an elevation in the chemokine CCL2. This inflammation is largely absent in GF mice, indicating the critical role of maternal microbes in mediating immune processes in utero. Furthermore, PNS in the absence of CCL2 failed to increase pro-inflammatory cytokine IL-6 in the fetal brain. PNS offspring also exhibited deficits in sociability and anxiety-like behavior that were absent in CCL2-/- PNS offspring. Tryptophan and serotonin (5-HT) were elevated in the WT PNS placenta, but not in CCL2-/- and GF animals. Altogether, these findings suggest that a complex interaction between maternal microbes, inflammation, and serotonin metabolism regulates the emergence of behavioral abnormalities following PNS.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Van den Bergh BRH, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav Rev. 2017;S0149-7634:30734–30735. - PubMed
-
- Gur TL, et al. Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain Behav. Immun. 2017;64:50–58. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 MH112892/MH/NIMH NIH HHS/United States
- KL2 TR001080/TR/NCATS NIH HHS/United States
- R21 MH117552/MH/NIMH NIH HHS/United States
- T32DE014320/U.S. Department of Health & Human Services | NIH | National Institute of Dental and Craniofacial Research (NIDCR)/International
- KL2 TR001068/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

