Response to Anti-HER2-Based Treatment in a Patient with Bladder Adenocarcinoma Harboring HER2 Amplification and S310F Mutation Discovered by Next-Generation Sequencing: A Case Report
- PMID: 32547059
- PMCID: PMC7244354
- DOI: 10.2147/OTT.S247515
Response to Anti-HER2-Based Treatment in a Patient with Bladder Adenocarcinoma Harboring HER2 Amplification and S310F Mutation Discovered by Next-Generation Sequencing: A Case Report
Abstract
Purpose: HER2 overexpression has been identified in approximately 14% of bladder adenocarcinomas. However, until now, there has been no approved standard targeted therapy for bladder adenocarcinoma patients harboring HER2 genetic alteration.
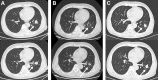
Case presentation: We presented a case of a 64-year-old man who was diagnosed with bladder adenocarcinoma, and lung metastasis was confirmed less than one year after initial bladder surgery. The patient received systemic chemotherapy and antiangiogenetic treatment, but the tumor continued to progress. The patient underwent next-generation sequencing (NGS) to seek potential treatment opportunities. HER2 amplification, approximately 7 times, was discovered together with the S310F mutation (mutant abundance 90%). The patient then received late-line treatment with trastuzumab and albumin-bound paclitaxel. A partial response was confirmed two months later. Trastuzumab-based therapy was continued for 8 cycles, and the progression-free survival period was 6 months. NGS was performed on a rebiopsy, and the result showed no amplification of HER2, and the S310F mutant abundance was reduced to 27.9%.
Conclusion: This is the first case report describing a bladder adenocarcinoma patient harboring HER2 amplification who responded to trastuzumab. NGS is of great potential in the selection of bladder adenocarcinoma patients suitable for anti-HER2 therapy. The genetic change after treatment also implied possible mechanisms of resistance to trastuzumab-based therapy, which requires more investigation.
Keywords: bladder adenocarcinoma; human epidermal growth factor receptor 2; next-generation sequencing; trastuzumab.
© 2020 Wang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest to disclose.
Figures

Similar articles
-
Response to Trastuzumab and Lapatinib in a Metastatic Colorectal Cancer Harboring HER2 Amplification and HER2 S310F Mutation.J Natl Compr Canc Netw. 2021 Jun 30;19(6):670-674. doi: 10.6004/jnccn.2021.7023. J Natl Compr Canc Netw. 2021. PMID: 34214965
-
Case Report: Effective Treatment With Pyrotinib and Capecitabine in a Heavily Pretreated Locally Advanced Breast Cancer Harboring Both HER2 Overexpression and Mutant.Front Oncol. 2021 Oct 14;11:715554. doi: 10.3389/fonc.2021.715554. eCollection 2021. Front Oncol. 2021. PMID: 34722261 Free PMC article.
-
A HER2-mutant patient with late-stage duodenal adenocarcinoma benefited from anti-HER2 therapy and PD-1 inhibition: a case report.J Gastrointest Oncol. 2021 Aug;12(4):1939-1943. doi: 10.21037/jgo-21-311. J Gastrointest Oncol. 2021. PMID: 34532140 Free PMC article.
-
A narrative review of advances in treatment and survival prognosis of HER2-positive malignant lung cancers.J Thorac Dis. 2021 Jun;13(6):3708-3720. doi: 10.21037/jtd-20-3265. J Thorac Dis. 2021. PMID: 34277062 Free PMC article. Review.
-
[Predictive diagnosis of HER2 in gastric adenocarcinoma].Cesk Patol. 2011 Oct;47(4):160-3. Cesk Patol. 2011. PMID: 22145214 Review. Czech.
Cited by
-
Interaction between HER2 and ATM predicts poor survival in bladder cancer patients.J Cell Mol Med. 2022 Oct;26(19):4959-4973. doi: 10.1111/jcmm.17512. Epub 2022 Sep 3. J Cell Mol Med. 2022. PMID: 36056635 Free PMC article.
-
Targeting HER2 genomic alterations in non-small cell lung cancer.J Natl Cancer Cent. 2021 May 3;1(2):58-73. doi: 10.1016/j.jncc.2021.04.001. eCollection 2021 Jun. J Natl Cancer Cent. 2021. PMID: 39035769 Free PMC article. Review.
-
Efficacy of targeted therapy in patients with HER2-positive non-small cell lung cancer: A systematic review and meta-analysis.Br J Clin Pharmacol. 2022 May;88(5):2019-2034. doi: 10.1111/bcp.15155. Epub 2021 Dec 21. Br J Clin Pharmacol. 2022. PMID: 34820879 Free PMC article.
-
Computational-aided rational mutation design of pertuzumab to overcome active HER2 mutation S310F through antibody-drug conjugates.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2413686122. doi: 10.1073/pnas.2413686122. Epub 2024 Dec 30. Proc Natl Acad Sci U S A. 2025. PMID: 39793038 Free PMC article.
References
-
- Lughezzani G, Sun M, Jeldres C, et al. Adenocarcinoma versus urothelial carcinoma of the urinary bladder: comparison between pathologic stage at radical cystectomy and cancer-specific mortality. Urology. 2010;75(2):381. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

