Mitochondrial Transcription Factor A added to Osteocytes in a Stressed Environment has a Cytoprotective Effect
- PMID: 32547324
- PMCID: PMC7294917
- DOI: 10.7150/ijms.45335
Mitochondrial Transcription Factor A added to Osteocytes in a Stressed Environment has a Cytoprotective Effect
Abstract
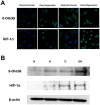
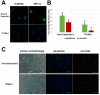
The main precipitant of glucocorticoid-associated femoral head osteonecrosis is widely accepted to be an ischemic-hypoxic event, with oxidative stress also as an underlying factor. Mitochondrial DNA is more vulnerable to oxidative injury than the nucleus, and mitochondrial transcription factor A (TFAM), which plays roles in its function, preservation, and regulation is being increasingly investigated. In the present study we focused on the impact of TFAM on the relation between the oxidative injury induced by the addition of glucocorticoid to a hypoxic environment and osteocytic cell necrosis. Using cultured osteocytes MLO-Y4 in a 1% hypoxic environment (hypoxia) to which 1µM dexamethasone (Dex) was added (Dex(+)/hypoxia(+)), an immunocytochemical study was conducted using 8-hydroxy-2'-deoxyguanosine (8-OHdG), an index of oxidative stress, and hypoxia inducible factor-1α (HIF-1α), a marker of hypoxia. Next, after adding TFAM siRNA, TFAM knockdown, cultured for 24h, and mitochondrial membrane potential were measured, they were stained with ATP5A which labels adenosine triphosphate (ATP) production. Dex was added to MLO-Y4 to which TFAM had been added, and cultured for 24h in hypoxia. The ratio of dead cells to viable cells was determined and compared. Enhanced expression of 8-OHdG, HIF-1α was found in osteocytes following the addition of glucocorticoid in a hypoxic environment. With TFAM knockdown, as compared to normoxia, mitochondrial function significantly decreased. On the other hand, by adding TFAM, the incidence of osteocytic cell necrosis was significantly decreased as compared with Dex(+)/hypoxia(+). TFAM was confirmed to be important in mitochondrial function and preservation, inhibition of oxidative injury and maintenance of ATP production. Moreover, prevention of mitochondrial injury can best be achieved by decreasing the development of osteocytic cell necrosis.
Keywords: mitochondrial function; mitochondrial transcription factor A (TFAM); osteocytic cell necrosis; oxidative injury.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997;40(11):2055–64. - PubMed
-
- Ichiseki T, Matsumoto T, Nishino M, Kaneuji A, Katsuda S. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J Orthop Sci. 2004;9(5):509–15. - PubMed
-
- Tsuji M, Ikeda H, Ishizu A, Miyatake Y, Hayase H, Yoshiki T. Altered expression of apoptosis-related genes in osteocytes exposed to high-dose steroid hormones and hypoxic stress. Pathobiology. 2006;73(6):304–9. - PubMed
-
- Zhu ZH, Gao YS, Zeng BF, Zhang CQ. The effect of dexamethasone and hypoxic stress on MC3T3-E1 cells. Front Biosci (Landmark Ed) 2011 Jun 1;16:2747–55. - PubMed
-
- Ueda S, Ichiseki T, Yoshitomi Y, Yonekura H, Ueda Y, Kaneuji A, Matsumoto T. Osteocytic cell necrosis is caused by a combination of glucocorticoid-induced Dickkopf-1 and hypoxia. Med Mol Morphol. 2015 Jun;48(2):69–75. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

