Neuronal Replacement as a Tool for Basal Ganglia Circuitry Repair: 40 Years in Perspective
- PMID: 32547369
- PMCID: PMC7272540
- DOI: 10.3389/fncel.2020.00146
Neuronal Replacement as a Tool for Basal Ganglia Circuitry Repair: 40 Years in Perspective
Abstract
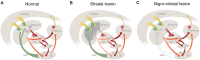
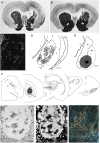
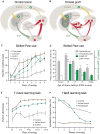
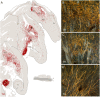
The ability of new neurons to promote repair of brain circuitry depends on their capacity to re-establish afferent and efferent connections with the host. In this review article, we give an overview of past and current efforts to restore damaged connectivity in the adult mammalian brain using implants of fetal neuroblasts or stem cell-derived neuronal precursors, with a focus on strategies aimed to repair damaged basal ganglia circuitry induced by lesions that mimic the pathology seen in humans affected by Parkinson's or Huntington's disease. Early work performed in rodents showed that neuroblasts obtained from striatal primordia or fetal ventral mesencephalon can become anatomically and functionally integrated into lesioned striatal and nigral circuitry, establish afferent and efferent connections with the lesioned host, and reverse the lesion-induced behavioral impairments. Recent progress in the generation of striatal and nigral progenitors from pluripotent stem cells have provided compelling evidence that they can survive and mature in the lesioned brain and re-establish afferent and efferent axonal connectivity with a remarkable degree of specificity. The studies of cell-based circuitry repair are now entering a new phase. The introduction of genetic and virus-based techniques for brain connectomics has opened entirely new possibilities for studies of graft-host integration and connectivity, and the access to more refined experimental techniques, such as chemo- and optogenetics, has provided new powerful tools to study the capacity of grafted neurons to impact the function of the host brain. Progress in this field will help to guide the efforts to develop therapeutic strategies for cell-based repair in Huntington's and Parkinson's disease and other neurodegenerative conditions involving damage to basal ganglia circuitry.
Keywords: dopamine; embryonic stem cells; induced pluripotent stem cells; nigrostriatal pathway; regenerative medicine; striatum; substantia nigra.
Copyright © 2020 Björklund and Parmar.
Figures

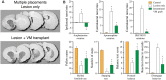

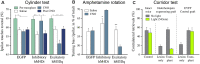
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

