Estrogen Formation and Inactivation Following TBI: What we Know and Where we Could go
- PMID: 32547495
- PMCID: PMC7272601
- DOI: 10.3389/fendo.2020.00345
Estrogen Formation and Inactivation Following TBI: What we Know and Where we Could go
Abstract
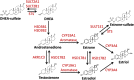
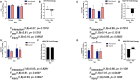
Traumatic brain injury (TBI) is responsible for various neuronal and cognitive deficits as well as psychosocial dysfunction. Characterized by damage inducing neuroinflammation, this response can cause an acute secondary injury that leads to widespread neurodegeneration and loss of neurological function. Estrogens decrease injury induced neuroinflammation and increase cell survival and neuroprotection and thus are a potential target for use following TBI. While much is known about the role of estrogens as a neuroprotective agent following TBI, less is known regarding their formation and inactivation following damage to the brain. Specifically, very little is known surrounding the majority of enzymes responsible for the production of estrogens. These estrogen metabolizing enzymes (EME) include aromatase, steroid sulfatase (STS), estrogen sulfotransferase (EST/SULT1E1), and some forms of 17β-hydroxysteroid dehydrogenase (HSD17B) and are involved in both the initial conversion and interconversion of estrogens from precursors. This article will review and offer new prospective and ideas on the expression of EMEs following TBI.
Keywords: HSD17B; TBI; androgen; aromatase; estrogen; sulfatase.
Copyright © 2020 Duncan.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

