Prolyl Oligopeptidase From Leishmania infantum: Biochemical Characterization and Involvement in Macrophage Infection
- PMID: 32547514
- PMCID: PMC7271538
- DOI: 10.3389/fmicb.2020.01060
Prolyl Oligopeptidase From Leishmania infantum: Biochemical Characterization and Involvement in Macrophage Infection
Abstract
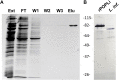
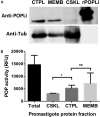
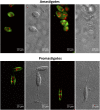
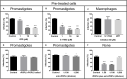
Leishmania infantum is a flagellated protozoan and one of the main causative agents of visceral leishmaniasis. This disease usually affects the human reticuloendothelial system, can cause death and available therapies may lead to serious side effects. Since it is a neglected tropical disease, the incentives for the development of new drugs are insufficient. It is important to know Leishmania virulence factors that contribute most to the disease in order to develop drugs. In the present work, we have produced L. infantum prolyl oligopeptidase (rPOPLi) in Escherichia coli, and investigated its biochemical properties as well as the effect of POP inhibitors on its enzymatic activity and on the inhibition of the macrophage infection by L. infantum. The optimal activity occurred at pH 7.5 and 37°C in the presence of DTT, the latter increased rPOPLi catalytic efficiency 5-fold on the substrate N-Suc-Gly-Pro-Leu-Gly-Pro-AMC. The enzyme was inhibited by TPCK, TLCK and by two POP specific inhibitors, Z-Pro-prolinal (ZPP, IC50 4.2 nM) and S17092 (IC50 3.5 nM). Besides being a cytoplasmic enzyme, POPLi is also found in punctuate structures within the parasite cytoplasm or associated with the parasite plasma membrane in amastigotes and promastigotes, respectively. Interestingly, S17092 and ZPP prevented parasite invasion in murine macrophages, supporting the involvement of POPLi in the invasive process of L. infantum. These data suggest POPLi as a virulence factor that offers potential as a target for designing new antileishmanial drugs.
Keywords: POPLi; drug target; leishmaniasis; protease; virulence factor.
Copyright © 2020 Lasse, Azevedo, de Araújo, Motta, Andrade, Rocha, Sampaio, Charneau, Gèze, Grellier, Santana and Bastos.
Figures


Similar articles
-
Leishmania infantum ecto-nucleoside triphosphate diphosphohydrolase-2 is an apyrase involved in macrophage infection and expressed in infected dogs.PLoS Negl Trop Dis. 2014 Nov 13;8(11):e3309. doi: 10.1371/journal.pntd.0003309. eCollection 2014 Nov. PLoS Negl Trop Dis. 2014. PMID: 25393008 Free PMC article.
-
Substrate-dependent, non-hyperbolic kinetics of pig brain prolyl oligopeptidase and its tight binding inhibition by JTP-4819.Biochem Pharmacol. 2002 Aug 1;64(3):463-71. doi: 10.1016/s0006-2952(02)01184-x. Biochem Pharmacol. 2002. PMID: 12147298
-
Activity and Cell-Death Pathway in Leishmania infantum Induced by Sugiol: Vectorization Using Yeast Cell Wall Particles Obtained From Saccharomyces cerevisiae.Front Cell Infect Microbiol. 2019 Jun 14;9:208. doi: 10.3389/fcimb.2019.00208. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31259161 Free PMC article.
-
Antileishmanial Activity, Cytotoxicity and Mechanism of Action of Clioquinol Against Leishmania infantum and Leishmania amazonensis Species.Basic Clin Pharmacol Toxicol. 2018 Sep;123(3):236-246. doi: 10.1111/bcpt.12990. Epub 2018 Apr 6. Basic Clin Pharmacol Toxicol. 2018. PMID: 29481714
-
An effective in vitro and in vivo antileishmanial activity and mechanism of action of 8-hydroxyquinoline against Leishmania species causing visceral and tegumentary leishmaniasis.Vet Parasitol. 2016 Feb 15;217:81-8. doi: 10.1016/j.vetpar.2016.01.002. Epub 2016 Jan 7. Vet Parasitol. 2016. PMID: 26827866
Cited by
-
Effects of a Serine Protease Inhibitor N-p-Tosyl-L-phenylalanine Chloromethyl Ketone (TPCK) on Leishmania amazonensis and Leishmania infantum.Pharmaceutics. 2022 Jun 29;14(7):1373. doi: 10.3390/pharmaceutics14071373. Pharmaceutics. 2022. PMID: 35890269 Free PMC article.
-
Three types of Leishmania mexicana amastigotes: Proteome comparison by quantitative proteomic analysis.Front Cell Infect Microbiol. 2022 Nov 9;12:1022448. doi: 10.3389/fcimb.2022.1022448. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439224 Free PMC article.
-
Profiling Serine Hydrolases in the Leishmania Host-Pathogen Interactome Using Cell-Permeable Activity-Based Fluorophosphonate Probes.Chembiochem. 2025 May 27;26(10):e202500160. doi: 10.1002/cbic.202500160. Epub 2025 May 14. Chembiochem. 2025. PMID: 40146885 Free PMC article.
-
Cryo-EM led analysis of open and closed conformations of Chagas vaccine candidate TcPOP.Nat Commun. 2025 Aug 5;16(1):7164. doi: 10.1038/s41467-025-62068-3. Nat Commun. 2025. PMID: 40764299 Free PMC article.
-
Discovery of Leishmania Druggable Serine Proteases by Activity-Based Protein Profiling.Front Pharmacol. 2022 Jul 15;13:929493. doi: 10.3389/fphar.2022.929493. eCollection 2022. Front Pharmacol. 2022. PMID: 35910377 Free PMC article.
References
-
- Bastos I. M., Grellier P., Martins N. F., Cadavid-Restrepo G., de Souza-Ault M. R., Augustyns K., et al. (2005). Molecular, functional and structural properties of the prolyl oligopeptidase of Trypanosoma cruzi (POP Tc80), which is required for parasite entry into mammalian cells. Biochem. J. 388 29–38. 10.1042/BJ20041049 - DOI - PMC - PubMed
-
- Bastos I. M., Motta F. N., Charneau S., Santana J. M., Dubost L., Augustyns K., et al. (2010). Prolyl oligopeptidase of Trypanosoma brucei hydrolyzes native collagen, peptide hormones and is active in the plasma of infected mice. Microb. Infect. 12 457–466. 10.1016/j.micinf.2010.02.007 - DOI - PubMed
LinkOut - more resources
Full Text Sources

