Adjuvant Effect of Toll-Like Receptor 9 Activation on Cancer Immunotherapy Using Checkpoint Blockade
- PMID: 32547560
- PMCID: PMC7274158
- DOI: 10.3389/fimmu.2020.01075
Adjuvant Effect of Toll-Like Receptor 9 Activation on Cancer Immunotherapy Using Checkpoint Blockade
Abstract
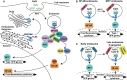
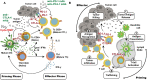
Immunotherapy using checkpoint blockade has revolutionized cancer treatment, improving patient survival and quality of life. Nevertheless, the clinical outcomes of such immunotherapy are highly heterogeneous between patients. Depending on the cancer type, the patient response rates to this immunotherapy are limited to 20-30%. Based on the mechanism underlying the antitumor immune response, new therapeutic strategies have been designed with the aim of increasing the effectiveness and specificity of the antitumor immune response elicited by checkpoint blockade agents. The activation of toll-like receptor 9 (TLR9) by its synthetic agonists induces the antitumor response within the innate immunity arm, generating adjuvant effects and priming the adaptive immune response elicited by checkpoint blockade during the effector phase of tumor-cell killing. This review first describes the underlying mechanisms of action and current status of monotherapy using TLR9 agonists and immune checkpoint inhibitors for cancer immunotherapy. The rationale for combining these two agents is discussed, and evidence indicating the current status of such combination therapy as a novel cancer treatment strategy is presented.
Keywords: CpG-ODN; adjuvant; cancer immunotherapy; immune checkpoint blockade; innate immune; toll-like receptor.
Copyright © 2020 Chuang, Tseng, Huang, Huang, Huang and Chuang.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

