Soil Sickness in Aged Tea Plantation Is Associated With a Shift in Microbial Communities as a Result of Plant Polyphenol Accumulation in the Tea Gardens
- PMID: 32547573
- PMCID: PMC7270330
- DOI: 10.3389/fpls.2020.00601
Soil Sickness in Aged Tea Plantation Is Associated With a Shift in Microbial Communities as a Result of Plant Polyphenol Accumulation in the Tea Gardens
Abstract
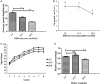
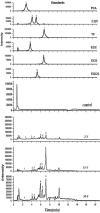
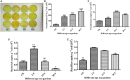
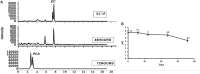
In conventional tea plantations, a large amount of pruned material returns to the soil surface, putting a high quantity of polyphenols into the soil. The accumulation of active allelochemicals in the tea rhizosphere and subsequent shift in beneficial microbes may be the cause of acidification, soil sickness, and regeneration problem, which may be attributed to hindrance of plant growth, development, and low yield in long-term monoculture tea plantation. However, the role of pruning leaf litter in soil sickness under consecutive tea monoculture is unclear. Here, we investigated soil samples taken from conventional tea gardens of different ages (2, 15, and 30 years) and under the effect of regular pruning. Different approaches including liquid chromatography-mass spectrometry (LC-MS) analysis of the leaf litter, metagenomic study of root-associated bacterial communities, and in vitro interaction of polyphenols with selected bacteria were applied to understand the effect of leaf litter-derived polyphenols on the composition and structure of the tea rhizosphere microbial community. Our results indicated that each pruning practice returns a large amount of leaf litter to each tea garden. LC-MS results showed that leaf litter leads to the accumulation of various allelochemicals in the tea rhizosphere, including epigallocatechin gallate, epigallocatechin, epicatechin gallate, catechin, and epicatechin with increasing age of the tea plantation. Meanwhile, in the tea garden grown consecutively for 30 years (30-Y), the phenol oxidase and peroxidase activities increased significantly. Pyrosequencing identified Burkholderia and Pseudomonas as the dominant genera, while plant growth-promoting bacteria, especially Bacillus, Prevotella, and Sphingomonas, were significantly reduced in the long-term tea plantation. The qPCR results of 30-Y soil confirmed that the copy numbers of bacterial genes per gram of the rhizosphere soil were significantly reduced, while that of Pseudomonas increased significantly. In vitro study showed that the growth of catechin-degrading bacteria (e.g., Pseudomonas) increased and plant-promoting bacteria (e.g., Bacillus) decreased significantly with increasing concentration of these allelochemicals. Furthermore, in vitro interaction showed a 0.36-fold decrease in the pH of the broth after 72 h with the catechin degradation. In summary, the increase of Pseudomonas and Burkholderia in the 30-Y garden was found to be associated with the accumulation of catechin substrates. In response to the long-term monoculture of tea, the variable soil pH along with the litter distribution negatively affect the population of plant growth-promoting bacteria (e.g., Sphingomonas, Bacillus, and Prevotella). Current research suggests that the removal of pruned branches from tea gardens can prevent soil sickness and may lead to sustainable tea production.
Keywords: catechins; indirect allelopathy; monoculture problems; plant polyphenol; soil sickness.
Copyright © 2020 Arafat, Ud Din, Tayyab, Jiang, Chen, Cai, Zhao, Lin, Lin and Lin.
Figures





References
-
- Acosta-Martínez V., Burow G., Zobeck T., Allen V. (2010). Soil microbial communities and function in alternative systems to continuous cotton. Soil Sci. Soc. Am. J. 74 1181–1192.
-
- Arafat Y., Tayyab M., Khan M. U., Chen T., Amjad H., Awais S., et al. (2019). Long-term monoculture negatively regulates Fungal community composition and abundance of Tea orchards. Agronomy 9:466.
-
- Baldwin I. T., Olson R. K., Reiners W. A. (1983). Protein binding phenolics and the inhibition of nitrification in subalpine balsam fir soils. Soil Biol. Biochem. 15 419–423.
LinkOut - more resources
Full Text Sources
Other Literature Sources

