Immune priming against bacteria in spiders and scorpions?
- PMID: 32547885
- PMCID: PMC7278890
- DOI: 10.7717/peerj.9285
Immune priming against bacteria in spiders and scorpions?
Abstract
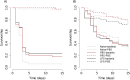
Empirical evidence of immune priming in arthropods keeps growing, both at the within- and trans-generational level. The evidence comes mostly from work on insects and it remains unclear for some other arthropods whether exposure to a non-lethal dose of a pathogen provides protection during a second exposure with a lethal dose. A poorly investigated group are arachnids, with regard to the benefits of immune priming measured as improved survival. Here, we investigated immune priming in two arachnids: the wolf spider Lycosa cerrofloresiana and the scorpion Centruroides granosus. We injected a third of the individuals with lipopolysaccharides of Escherichia coli (LPS, an immune elicitor), another third were injected with the control solution (PBS) and the other third were kept naive. Four days after the first inoculations, we challenged half of the individuals of each group with an injection of a high dose of E. coli and the other half was treated with the control solution. For scorpions, individuals that were initially injected with PBS or LPS did not differ in their survival rates against the bacterial challenge. Individuals injected with LPS showed higher survival than that of naive individuals as evidence of immune priming. Individuals injected with PBS tended to show higher survival rates than naive individuals, but the difference was not significant-perhaps suggesting a general immune upregulation caused by the wounding done by the needle. For spiders, we did not observe evidence of priming, the bacterial challenge reduced the survival of naive, PBS and LPS individuals at similar rates. Moreover; for scorpions, we performed antibacterial assays of hemolymph samples from the three priming treatments (LPS, PBS and naive) and found that the three treatments reduced bacterial growth but without differences among treatments. As non-model organisms, with some unique differences in their immunological mechanisms as compared to the most studied arthropods (insects), arachnids provide an unexplored field to elucidate the evolution of immune systems.
Keywords: Escherichia coli; Immune priming; LPS; Scorpion; Spider; Survival.
©2020 Gálvez et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

References
-
- Adamo SA. How should behavioural ecologists interpret measurements of immunity? Animal Behaviour. 2004;68:1443–1449. doi: 10.1016/j.anbehav.2004.05.005. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

