Direct production of fatty alcohols from glucose using engineered strains of Yarrowia lipolytica
- PMID: 32547923
- PMCID: PMC7283507
- DOI: 10.1016/j.mec.2019.e00105
Direct production of fatty alcohols from glucose using engineered strains of Yarrowia lipolytica
Erratum in
-
Erratum regarding previously published articles in volumes 9, 10 and 11.Metab Eng Commun. 2021 Oct 28;13:e00186. doi: 10.1016/j.mec.2021.e00186. eCollection 2021 Dec. Metab Eng Commun. 2021. PMID: 34765440 Free PMC article.
Abstract
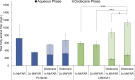
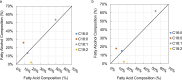
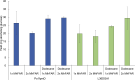
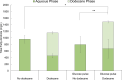
Fatty alcohols are important industrial oleochemicals with broad applications and a growing market. Here, we sought to engineer Yarrowia lipolytica to serve as a renewable source of fatty alcohols (specifically hexadecanol, heptadecanol, octadecanol, and oleyl alcohol) directly from glucose. Through screening four fatty acyl-CoA reductase (FAR) enzyme variants across two engineered background strains, we identified that MhFAR enabled the highest production. Further strain engineering, fed-batch flask cultivation, and extractive fermentation improved the fatty alcohol titer to 1.5 g/L. Scale-up of this strain in a 2L bioreactor led to 5.8 g/L total fatty alcohols at an average yield of 36 mg/g glucose with a maximum productivity of 39 mg/L hr. Finally, we utilized this fatty alcohol reductase to generate a customized fatty alcohol, linolenyl alcohol, from α-linolenic acid. Overall, this work demonstrates Y. lipolytica is a robust chassis for diverse fatty alcohol production and highlights the capacity to obtain high titers and yields from a purely minimal media formulation directly from glucose without the need for complex additives.
Keywords: Extractive fermentation; Fatty acyl-CoA reductase; Fatty alcohols; Product yield; Yarrowia lipolytica.
© 2019 The Authors.
Figures


References
-
- Abdel-Mawgoud A.M. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018;50:192–208. - PubMed
-
- Adrio J.L. Oleaginous yeasts: promising platforms for the production of oleochemicals and biofuels. Biotechnol. Bioeng. 2017;114(9):1915–1920. - PubMed
-
- Blazeck J. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab. Eng. 2015;32:66–73. - PubMed
-
- Blazeck J. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat. Commun. 2014;5 - PubMed
-
- Blazeck J. Generalizing a hybrid synthetic promoter approach in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2013;97 - PubMed
LinkOut - more resources
Full Text Sources

