The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders
- PMID: 32547962
- PMCID: PMC7270209
- DOI: 10.3389/fcimb.2020.00248
The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders
Abstract
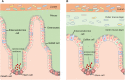
Mucus is integral to gut health and its properties may be affected in neurological disease. Mucus comprises a hydrated network of polymers including glycosylated mucin proteins. We propose that factors that influence the nervous system may also affect the volume, viscosity, porosity of mucus composition and subsequently, gastrointestinal (GI) microbial populations. The gut has its own intrinsic neuronal network, the enteric nervous system, which extends the length of the GI tract and innervates the mucosal epithelium. The ENS regulates gut function including mucus secretion and renewal. Both dysbiosis and gut dysfunction are commonly reported in several neurological disorders such as Parkinson's and Alzheimer's disease as well in patients with neurodevelopmental disorders including autism. Since some microbes use mucus as a prominent energy source, changes in mucus properties could alter, and even exacerbate, dysbiosis-related gut symptoms in neurological disorders. This review summarizes existing knowledge of the structure and function of the mucus of the GI tract and highlights areas to be addressed in future research to better understand how intestinal homeostasis is impacted in neurological disorders.
Keywords: MUC-2; goblet cells; intestine; microbes; mucus; neurological disorders.
Copyright © 2020 Herath, Hosie, Bornstein, Franks and Hill-Yardin.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

