Decreased Outpatient Fluoroquinolone Prescribing Using a Multimodal Antimicrobial Stewardship Initiative
- PMID: 32548204
- PMCID: PMC7284006
- DOI: 10.1093/ofid/ofaa182
Decreased Outpatient Fluoroquinolone Prescribing Using a Multimodal Antimicrobial Stewardship Initiative
Abstract
Background: Fluoroquinolones are antibiotics prescribed in the outpatient setting, though they have serious side effects. This study evaluates the impact of stewardship interventions on total and inappropriate prescribing of fluoroquinolones in outpatient settings in a large county hospital and health system.
Methods: In an effort to decrease inappropriate outpatient fluoroquinolone usage, a multimodal antimicrobial stewardship initiative was implemented in November 2016. Education regarding the risks, benefits, and appropriate uses of fluoroquinolones was provided to providers in different outpatient settings, Food and Drug Administration warnings were added to all oral fluoroquinolone orders, an outpatient order set for cystitis treatment was created, and fluoroquinolone susceptibilities were suppressed when appropriate. Charts from October 2016, 2017, and 2018 were retrospectively reviewed if the patient encounter occurred in primary care clinics, emergency departments, or urgent care centers within Parkland Health & Hospital System and a fluoroquinolone was prescribed. Inappropriate use was defined as a fluoroquinolone prescription for cystitis, bronchitis, or sinusitis in a patient without a history of Pseudomonas aeruginosa or multidrug-resistant organisms and without drug allergies that precluded use of other oral antibiotics.
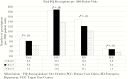
Results: Total fluoroquinolone prescriptions per 1000 patient visits decreased significantly by 39% (P < .01), and inappropriate fluoroquinolone use decreased from 53% to 34% (P < .01). More than 90% of inappropriate fluoroquinolone prescriptions were given for cystitis, while bronchitis and sinusitis accounted for only 4.4% and 1.6% of inappropriate indications, respectively.
Conclusion: A multimodal stewardship initiative appears to effectively reduce both total and inappropriate outpatient fluoroquinolone prescriptions.
Keywords: antimicrobial stewardship; fluoroquinolone; outpatient stewardship.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- US Food and Drug Administration. Information for healthcare professionals: fluoroquinolone antimicrobial drugs [ciprofloxacin (marketed as Cipro and generic ciprofloxacin), ciprofloxacin extended-release (marketed as Cipro XR and Proquin XR), gemifloxacin (marketed as Factive), levofloxacin (marketed as Levaquin), moxifloxacin (marketed as Avelox), norfloxacin (marketed as Noroxin), and ofloxacin (marketed as Floxin)].2008 Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa.... Accessed 21 July 2018.
-
- US Food and Drug Administration. FDA Drug Safety Communication: FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection. News release.2013 Available at: http://www.fda.gov/Drugs/DrugSafety/ucm365050.htm. Accessed 21 July 2018.
-
- US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. News release. 2016 Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c.... Accessed 21 July 2018.
-
- US Food and Drug Administration. FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. News release.2018 Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm612995.htm. Accessed 21 July 2018.