Glucose transporter 1 is important for the glycolytic metabolism of human endometrial stromal cells in hypoxic environment
- PMID: 32548315
- PMCID: PMC7286975
- DOI: 10.1016/j.heliyon.2020.e03985
Glucose transporter 1 is important for the glycolytic metabolism of human endometrial stromal cells in hypoxic environment
Abstract
Aim: The study aimed to elucidate the glycolytic metabolism of human endometrial stromal cells (hESCs) in hypoxic environment.
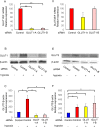
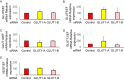
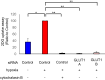
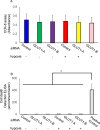
Main methods: The hESCs were cultured in hypoxic environment, and their metabolic pathways were analyzed using metabolomics. We assessed glucose uptake using 2-deoxyglucose (2-DG) assay. The expression of glucose transporters (GLUTs) required for glucose uptake was determined using real-time quantitative polymerase chain reaction (qPCR) and western blotting. Furthermore, we knocked down GLUT1 and examined the uptake of 2-DG.
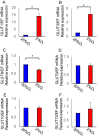
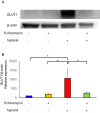
Key findings: Under hypoxia, glucose-6-phosphate, fructose-6-phosphate, and fructose-1,6-diphosphate were significantly elevated in hESCs (P < 0.05). This finding indicated enhancement in glycolysis. The volume of glucose uptake increased significantly under hypoxia (P < 0.05). Hypoxia simultaneously induced the expression of GLUT1 and GLUT3 mRNA (P < 0.05) and attenuated the expression of GLUT8 (P < 0.05). Glucose uptake was significantly inhibited upon knockdown of GLUT1 (P < 0.0001).
Significance: These results demonstrated a very important role of glucose transport under hypoxia. Also, hESCs utilize glycolysis to adapt to hypoxic conditions that could occur in menstrual and implantation period. These findings pave the way to study implantation failure and tumors originating from the endometrium.
Keywords: Biological sciences; Cell biology; Cell culture; Endometrium; Glucose transporter; Glycolysis; Human endometrial stromal cells; Hypoxia; Metabolomics; Molecular biology; Oxidative stress; Reproductive system; Women's health.
© 2020 The Authors.
Figures


Similar articles
-
Hypoxic preconditioning up-regulates glucose transport activity and glucose transporter (GLUT1 and GLUT3) gene expression after acute anoxic exposure in the cultured rat hippocampal neurons and astrocytes.Brain Res. 2008 May 23;1211:22-9. doi: 10.1016/j.brainres.2005.04.029. Brain Res. 2008. PMID: 18474279
-
Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers.Pathol Oncol Res. 2012 Jul;18(3):721-8. doi: 10.1007/s12253-012-9500-5. Epub 2012 Jan 21. Pathol Oncol Res. 2012. PMID: 22270867 Free PMC article.
-
Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy.J Clin Endocrinol Metab. 2003 Aug;88(8):3885-92. doi: 10.1210/jc.2002-021890. J Clin Endocrinol Metab. 2003. PMID: 12915684
-
Endometrial Glucose Transporters in Health and Disease.Front Cell Dev Biol. 2021 Sep 6;9:703671. doi: 10.3389/fcell.2021.703671. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34552924 Free PMC article. Review.
-
Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics.Chemotherapy. 2007;53(4):233-56. doi: 10.1159/000104457. Epub 2007 Jun 25. Chemotherapy. 2007. PMID: 17595539 Review.
Cited by
-
FSH mediates estradiol synthesis in hypoxic granulosa cells by activating glycolytic metabolism through the HIF-1α-AMPK-GLUT1 signaling pathway.J Biol Chem. 2022 May;298(5):101830. doi: 10.1016/j.jbc.2022.101830. Epub 2022 Mar 15. J Biol Chem. 2022. PMID: 35300979 Free PMC article.
-
An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors.Front Pharmacol. 2022 Nov 1;13:1035510. doi: 10.3389/fphar.2022.1035510. eCollection 2022. Front Pharmacol. 2022. PMID: 36386187 Free PMC article. Review.
-
Hereditary Leiomyomatosis and Renal Cell Cancer: Recent Insights Into Mechanisms and Systemic Treatment.Front Oncol. 2021 May 25;11:686556. doi: 10.3389/fonc.2021.686556. eCollection 2021. Front Oncol. 2021. PMID: 34113573 Free PMC article. Review.
-
Exposure to cigarette smoke affects endometrial maturation including angiogenesis and decidualization.Reprod Med Biol. 2021 Jan 11;20(1):108-118. doi: 10.1002/rmb2.12360. eCollection 2021 Jan. Reprod Med Biol. 2021. PMID: 33488290 Free PMC article.
-
The Regulators of Human Endometrial Stromal Cell Decidualization.Biomolecules. 2022 Sep 10;12(9):1275. doi: 10.3390/biom12091275. Biomolecules. 2022. PMID: 36139114 Free PMC article. Review.
References
-
- Critchley H.O., Osei J., Henderson T.A., Boswell L., Sales K.J., Jabbour H.N. Hypoxia-inducible factor-1α expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2) Endocrinology. 2006;147:744–753. - PubMed
-
- Tsuzuki T., Okada H., Cho H., Tsuji S., Nishigaki A., Yasuda K. Hypoxic stress simultaneously stimulates vascular endothelial growth factor via hypoxia-inducible factor-1α and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum. Reprod. 2012;27:523–530. - PubMed
-
- West J.B. Physiological effects of chronic hypoxia. N. Engl. J. Med. 2017;376:1965–1971. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

