Glucose Oxidase Immobilized on Magnetic Zirconia: Controlling Catalytic Performance and Stability
- PMID: 32548416
- PMCID: PMC7271398
- DOI: 10.1021/acsomega.0c01067
Glucose Oxidase Immobilized on Magnetic Zirconia: Controlling Catalytic Performance and Stability
Abstract
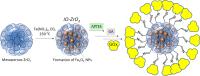
Here, we report the structures and properties of biocatalysts based on glucose oxidase (GOx) macromolecules immobilized on the mesoporous zirconia surface with or without magnetic iron oxide nanoparticles (IONPs) in zirconia pores. Properties of these biocatalysts were studied in oxidation of d-glucose to d-gluconic acid at a wide range of pH and temperatures. We demonstrate that the calcination temperature (300, 400, or 600 °C) of zirconia determines its structure, with crystalline materials obtained at 400 and 600 °C. This, in turn, influences the catalytic behavior of immobilized GOx, which was tentatively assigned to the preservation of GOx conformation on the crystalline support surface. IONPs significantly enhance the biocatalyst activity due to synergy with the enzyme. At the same time, neither support porosity nor acidity/basicity shows correlations with the properties of this biocatalyst. The highest relative activity of 98% (of native GOx) at a pH 6-7 and temperature of 40-45 °C was achieved for the biocatalyst based on ZrO2 calcined at 600 °C and containing IONPs. This process is green as it is characterized by a high atom economy due to the formation of a single product with high selectivity and conversion and minimization of waste due to magnetic separation of the catalyst from an aqueous solution. These and an exceptional stability of this catalyst in 10 consecutive reactions (7% relative activity loss) make it favorable for practical applications.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Drout R. J.; Robison L.; Farha O. K. Catalytic applications of enzymes encapsulated in metal-organic frameworks. Coord. Chem. Rev. 2019, 381, 151–160. 10.1016/j.ccr.2018.11.009. - DOI
-
- Cui J.; Ren S.; Sun B.; Jia S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41. 10.1016/j.ccr.2018.05.004. - DOI
LinkOut - more resources
Full Text Sources

