Derivatives of a PARP Inhibitor TIQ-A through the Synthesis of 8-Alkoxythieno[2,3- c]isoquinolin-5(4 H)-ones
- PMID: 32548533
- PMCID: PMC7288715
- DOI: 10.1021/acsomega.0c01879
Derivatives of a PARP Inhibitor TIQ-A through the Synthesis of 8-Alkoxythieno[2,3- c]isoquinolin-5(4 H)-ones
Abstract
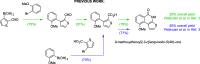
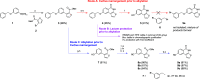
Thieno[2,3-c]isoquinolin-5(4H)-one is known for its potential as an anti-ischemic agent through the inhibition of poly(ADP-ribose) polymerase 1 (PARP1). However, the compound also inhibits many other enzymes of the PARP family, potentially limiting its usability. The broad inhibition profile, on the other hand, indicates that this molecule backbone could be potentially used as a scaffold for the development of specific inhibitors for certain PARP enzymes. These efforts call for novel synthetic strategies for substituted thieno[2,3-c]isoquinolin-5(4H)-one that could provide the needed selectivity. In this article, an efficient synthetic strategy for 8-alkoxythieno[2,3-c]isoquinolin-5(4H)-ones through eight steps is presented and other tested synthetic pathways are discussed in detail. Synthesis of 7-methoxythieno[2,3-c]isoquinolin-5(4H)-one is also demonstrated to show that the strategy can be applied widely in the syntheses of substituted alkoxythieno[2,3-c]isoquinolin-5(4H)-ones.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

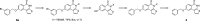
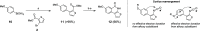
References
-
- Chiarugi A.; Meli E.; Calvani M.; Picca R.; Baronti R.; Camaioni E.; Costantino G.; Marinozzi M.; Pellegrini-Giampietro D. E.; Pellicciari R.; Moroni F. Novel isoquinolinone-derived inhibitors of poly(ADP-ribose) polymerase-1: Pharmacological characterization and neuroprotective effects in an in vitro model of cerebral ischemia. J. Pharmacol. Exp. Ther. 2003, 305, 943–949. 10.1124/jpet.103.048934. - DOI - PubMed
-
- Pellicciari R.; Camaioni E.; Costantino G.; Marinozzi M.; Macchiarulo A.; Moroni F.; Natalini B. Towards new neuroprotective agents: design and synthesis of 4H-thieno[2,3-c] isoquinolin-5-one derivatives as potent PARP-1 inhibitors. Il Farmaco 2003, 58, 851–858. 10.1016/S0014-827X(03)00143-5. - DOI - PubMed
-
- Pellicciari R.; Camaioni E.; Gilbert A. M.; Macchiarulo A.; Bikker J. A.; Shah F.; Bard J.; Costantino G.; Gioiello A.; Robertson G. M.; Sabbatini P.; Venturoni F.; Liscio P.; Carotti A.; Bellocchi D.; Cozzi A.; Wood A.; Gonzales C.; Zaleska M. M.; Ellingboe J. W.; Moroni F. Discovery and characterization of novel potent PARP-1 inhibitors endowed with neuroprotective properties: From TIQ-A to HYDAMTIQ. Med. Chem. Commun. 2011, 2, 559–565. 10.1039/c1md00021g. - DOI
-
- Moroni F.; Cozzi A.; Chiarugi A.; Formentini L.; Camaioni E.; Pellegrini-Giampietro D. E.; Chen Y.; Liang S.; Zaleska M. M.; Gonzales C.; Wood A.; Pellicciari R. Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br. J. Pharmacol. 2012, 165, 1487–1500. 10.1111/j.1476-5381.2011.01666.x. - DOI - PMC - PubMed
-
- Marchand J.-R.; Carotti A.; Passeri D.; Filipponi P.; Liscio P.; Camaioni E.; Pellicciari R.; Gioiello A.; Macchiarulo A. Investigating the allosteric reverse signalling of PARP inhibitors withmicrosecond molecular dynamic simulations andfluorescence anisotropy. Biochim. Biophys. Acta, Proteins Proteomics 2014, 1844, 1765–1772. 10.1016/j.bbapap.2014.07.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

