Long-term clinical outcomes of losartan in patients with Marfan syndrome: follow-up of the multicentre randomized controlled COMPARE trial
- PMID: 32548624
- PMCID: PMC7711887
- DOI: 10.1093/eurheartj/ehaa377
Long-term clinical outcomes of losartan in patients with Marfan syndrome: follow-up of the multicentre randomized controlled COMPARE trial
Abstract
Aims: The COMPARE trial showed a small but significant beneficial effect of 3-year losartan treatment on aortic root dilatation rate in adults with Marfan syndrome (MFS). However, no significant effect was found on clinical endpoints, possibly due to a short follow-up period. The aim of the current study was therefore to investigate the long-term clinical outcomes after losartan treatment.
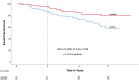
Methods and results: In the original COMPARE study (inclusion 2008-2009), adult patients with MFS (n = 233) were randomly allocated to either the angiotensin-II receptor blocker losartan® on top of regular treatment (β-blockers in 71% of the patients) or no additional medication. After the COMPARE trial period of 3 years, study subjects chose to continue their losartan medication or not. In a median follow-up period of 8 years, 75 patients continued losartan medication, whereas 78 patients, originally allocated to the control group, never used losartan after inclusion. No differences existed between baseline characteristics of the two groups except for age at inclusion [losartan 34 (interquartile range, IQR 26-43) years, control 41 (IQR 30-52) years; P = 0.031], and β-blocker use (losartan 81%, control 64%; P = 0.022). A pathological FBN1 mutation was present in 76% of patients and 58% of the patients were male. Clinical endpoints, defined as all-cause mortality, aortic dissection/rupture, elective aortic root replacement, reoperation, and vascular graft implantation beyond the aortic root, were compared between the two groups. A per-patient composite endpoint was also analysed. Five deaths, 14 aortic dissections, 23 aortic root replacements, 3 reoperations, and 3 vascular graft implantations beyond the aortic root occurred during follow-up. Except for aortic root replacement, all endpoints occurred in patients with an operated aortic root. Patients who used losartan during the entire follow-up period showed a reduced number of events compared to the control group (death: 0 vs. 5, P = 0.014; aortic dissection: 3 vs. 11, P = 0.013; elective aortic root replacement: 10 vs. 13, P = 0.264; reoperation: 1 vs. 2, P = 0.463; vascular graft implantations beyond the aortic root 0 vs. 3, P = 0.071; and composite endpoint: 14 vs. 26, P = 0.019). These results remained similar when corrected for age and β-blocker use in a multivariate analysis.
Conclusion: These results suggest a clinical benefit of combined losartan and β-blocker treatment in patients with MFS.
Keywords: Angiotensin-II receptor blocker; Losartan; Marfan syndrome; β-blocker.
© The Author(s) 2020. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Comment in
-
Marfan sartan saga, episode X.Eur Heart J. 2020 Nov 14;41(43):4188-4190. doi: 10.1093/eurheartj/ehaa418. Eur Heart J. 2020. PMID: 32607591 No abstract available.
References
-
- Murdoch JL, Walker BA, Halpern BL, Kuzma JW, McKusick VA.. Life expectancy and causes of death in the Marfan syndrome. N Engl J Med 1972;286:804–808. - PubMed
-
- Finkbohner R, Johnston D, Crawford ES, Coselli J, Milewicz DM.. Marfan syndrome. Long-term survival and complications after aortic aneurysm repair. Circulation 1995;91:728–733. - PubMed
-
- Silverman DI, Burton KJ, Gray J, Bosner MS, Kouchoukos NT, Roman MJ, Boxer M, Devereux RB, Tsipouras P.. Life expectancy in the Marfan syndrome. Am J Cardiol 1995;75:157–160. - PubMed
-
- Selamet Tierney ES, Feingold B, Printz BF, Park SC, Graham D, Kleinman CS, Mahnke CB, Timchak DM, Neches WH, Gersony WM.. Beta-blocker therapy does not alter the rate of aortic root dilation in pediatric patients with Marfan syndrome. J Pediatr 2007;150:77–82. - PubMed
-
- Ladouceur M, Fermanian C, Lupoglazoff JM, Edouard T, Dulac Y, Acar P, Magnier S, Jondeau G.. Effect of beta-blockade on ascending aortic dilatation in children with the Marfan syndrome. Am J Cardiol 2007;99:406–409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

