The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and their Application as a Degradable Scaffold for Organoid Passaging
- PMID: 32548863
- PMCID: PMC7669673
- DOI: 10.1002/adma.201905366
The Effect of Thiol Structure on Allyl Sulfide Photodegradable Hydrogels and their Application as a Degradable Scaffold for Organoid Passaging
Abstract
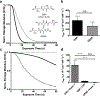
Intestinal organoids are useful in vitro models for basic and translational studies aimed at understanding and treating disease. However, their routine culture relies on animal-derived matrices that limit translation to clinical applications. In fact, there are few fully defined, synthetic hydrogel systems that allow for the expansion of intestinal organoids. Here, an allyl sulfide photodegradable hydrogel is presented, achieving rapid degradation through radical addition-fragmentation chain transfer (AFCT) reactions, to support routine passaging of intestinal organoids. Shear rheology to first characterize the effect of thiol and allyl sulfide crosslink structures on degradation kinetics is used. Irradiation with 365 nm light (5 mW cm-2 ) in the presence of a soluble thiol (glutathione at 15 × 10-3 m), and a photoinitiator (lithium phenyl-2,4,6-trimethylbenzoylphosphinate at 1 × 10-3 m), leads to complete hydrogel degradation in less than 15 s. Allyl sulfide hydrogels are used to support the formation of epithelial colonies from single intestinal stem cells, and rapid photodegradation is used to achieve repetitive passaging of stem cell colonies without loss in morphology or organoid formation potential. This platform could support long-term culture of intestinal organoids, potentially replacing the need for animal-derived matrices, while also allowing systematic variations to the hydrogel properties tailored for the organoid of interest.
Keywords: intestinal organoids; photodegradable hydrogels; tissue engineering.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

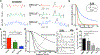




References
-
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y, Nature 2011, 472, 51. - PubMed
-
- Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, Wu MZ, Dubova I, Esteban CR, Montserrat N, Campistol JM, Izpisua Belmonte JC, Nat. Cell Biol 2013, 15, 1507. - PubMed
-
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL, Cell 2016, 165, 1238. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

