Is aquaporin-3 involved in water-permeability changes in the killifish during hypoxia and normoxic recovery, in freshwater or seawater?
- PMID: 32548921
- PMCID: PMC7405550
- DOI: 10.1002/jez.2393
Is aquaporin-3 involved in water-permeability changes in the killifish during hypoxia and normoxic recovery, in freshwater or seawater?
Abstract
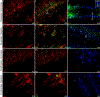
Aquaporins are the predominant water-transporting proteins in vertebrates, but only a handful of studies have investigated aquaporin function in fish, particularly in mediating water permeability during salinity challenges. Even less is known about aquaporin function in hypoxia (low oxygen), which can profoundly affect gill function. Fish deprived of oxygen typically enlarge gill surface area and shrink the water-to-blood diffusion distance, to facilitate oxygen uptake into the bloodstream. However, these alterations to gill morphology can result in unfavorable water and ion fluxes. Thus, there exists an osmorespiratory compromise, whereby fish must try to balance high branchial gas exchange with low ion and water permeability. Furthermore, the gills of seawater and freshwater teleosts have substantially different functions with respect to osmotic and ion fluxes; consequently, hypoxia can have very different effects according to the salinity of the environment. The purpose of this study was to determine what role aquaporins play in water permeability in the hypoxia-tolerant euryhaline common killifish (Fundulus heteroclitus), in two important osmoregulatory organs-the gills and intestine. Using immunofluorescence, we localized aquaporin-3 (AQP3) protein to the basolateral and apical membranes of ionocytes and enterocytes, respectively. Although hypoxia increased branchial AQP3 messenger-RNA expression in seawater and freshwater, protein abundance did not correlate. Indeed, hypoxia did not alter AQP3 protein abundance in seawater and reduced it in the cell membranes of freshwater gills. Together, these observations suggest killifish AQP3 contributes to reduced diffusive water flux during hypoxia and normoxic recovery in freshwater and facilitates intestinal permeability in seawater and freshwater.
Keywords: Fundulus heteroclitus; gills; immunofluorescence; intestine; mRNA; osmorespiratory compromise; oxygen deprivation; permeability; protein; western blot.
© 2020 Wiley Periodicals LLC.
Figures




Similar articles
-
Expression of aquaporin 3 in gills of the Atlantic killifish (Fundulus heteroclitus): Effects of seawater acclimation.Comp Biochem Physiol A Mol Integr Physiol. 2012 Mar;161(3):320-6. doi: 10.1016/j.cbpa.2011.11.014. Epub 2011 Dec 13. Comp Biochem Physiol A Mol Integr Physiol. 2012. PMID: 22193757 Free PMC article.
-
Ionoregulatory aspects of the hypoxia-induced osmorespiratory compromise in the euryhaline Atlantic killifish (Fundulus heteroclitus): the effects of salinity.J Exp Biol. 2020 Jul 13;223(Pt 13):jeb216309. doi: 10.1242/jeb.216309. J Exp Biol. 2020. PMID: 32487667
-
The osmorespiratory compromise in the euryhaline killifish: water regulation during hypoxia.J Exp Biol. 2019 Sep 24;222(Pt 18):jeb204818. doi: 10.1242/jeb.204818. J Exp Biol. 2019. PMID: 31466998 Free PMC article.
-
The osmorespiratory compromise in the fish gill.Comp Biochem Physiol A Mol Integr Physiol. 2021 Apr;254:110895. doi: 10.1016/j.cbpa.2021.110895. Epub 2021 Jan 8. Comp Biochem Physiol A Mol Integr Physiol. 2021. PMID: 33429056 Review.
-
Linking environmental salinity to respiratory phenotypes and metabolic rate in fishes: a data mining and modelling approach.J Exp Biol. 2022 Mar 8;225(Suppl_1):jeb243421. doi: 10.1242/jeb.243421. Epub 2022 Mar 8. J Exp Biol. 2022. PMID: 35258603 Review.
Cited by
-
The digestive tract as an essential organ for water acquisition in marine teleosts: lessons from euryhaline eels.Zoological Lett. 2021 Jun 21;7(1):10. doi: 10.1186/s40851-021-00175-x. Zoological Lett. 2021. PMID: 34154668 Free PMC article. Review.
-
De novo genome hybrid assembly and annotation of the endangered and euryhaline fish Aphanius iberus (Valenciennes, 1846) with identification of genes potentially involved in salinity adaptation.BMC Genomics. 2025 Feb 12;26(1):136. doi: 10.1186/s12864-025-11327-0. BMC Genomics. 2025. PMID: 39939939 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

