Synthesis and Antimicrobial Studies of Coumarin-Substituted Pyrazole Derivatives as Potent Anti- Staphylococcus aureus Agents
- PMID: 32549248
- PMCID: PMC7356691
- DOI: 10.3390/molecules25122758
Synthesis and Antimicrobial Studies of Coumarin-Substituted Pyrazole Derivatives as Potent Anti- Staphylococcus aureus Agents
Abstract
In this paper, synthesis and antimicrobial studies of 31 novel coumarin-substituted pyrazole derivatives are reported. Some of these compounds have shown potent activity against methicillin-resistant Staphylococcus aureus (MRSA) with minimum inhibitory concentration (MIC) as low as 3.125 µg/mL. These molecules are equally potent at inhibiting the development of MRSA biofilm and the destruction of preformed biofilm. These results are very significant as MRSA strains have emerged as one of the most menacing pathogens of humans and this bacterium is bypassing HIV in terms of fatality rate.
Keywords: MRSA; S. epidermidis; Staphylococcus aureus; antimicrobial; biofilm; coumarin; hydrazone; pyrazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




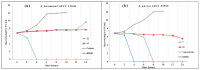
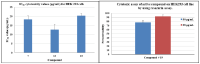
References
-
- WHO New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. [(accessed on 28 February 2020)]; Available online: https://www.who.int/news-room/detail/29-04-2019-new-report-calls-for-urg....
-
- CDC Antibiotic/Antimicrobial Resistance (AR/AMR) [(accessed on 28 February 2020)]; Available online: https://www.cdc.gov/drugresistance/biggest-threats.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

