Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis
- PMID: 32549301
- PMCID: PMC7353222
- DOI: 10.3390/nu12061780
Risk of Adverse Outcomes in Females Taking Oral Creatine Monohydrate: A Systematic Review and Meta-Analysis
Abstract
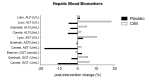
Creatine Monohydrate (CrM) is a dietary supplement routinely used as an ergogenic aid for sport and training, and as a potential therapeutic aid to augment different disease processes. Despite its increased use in recent years, studies reporting potential adverse outcomes of CrM have been mostly derived from male or mixed sex populations. A systematic search was conducted, which included female participants on CrM, where adverse outcomes were reported, with meta-analysis performed where appropriate. Six hundred and fifty-six studies were identified where creatine supplementation was the primary intervention; fifty-eight were female only studies (9%). Twenty-nine studies monitored for adverse outcomes, with 951 participants. There were no deaths or serious adverse outcomes reported. There were no significant differences in total adverse events, (risk ratio (RR) 1.24 (95% CI 0.51, 2.98)), gastrointestinal events, (RR 1.09 (95% CI 0.53, 2.24)), or weight gain, (mean difference (MD) 1.24 kg pre-intervention, (95% CI -0.34, 2.82)) to 1.37 kg post-intervention (95% CI -0.50, 3.23)), in CrM supplemented females, when stratified by dosing regimen and subject to meta-analysis. No statistically significant difference was reported in measures of renal or hepatic function. In conclusion, mortality and serious adverse events are not associated with CrM supplementation in females. Nor does the use of creatine supplementation increase the risk of total adverse outcomes, weight gain or renal and hepatic complications in females. However, all future studies of creatine supplementation in females should consider surveillance and comprehensive reporting of adverse outcomes to better inform participants and health professionals involved in future trials.
Keywords: adverse outcomes; creatine monohydrate; female; human; safety; supplementation.
Conflict of interest statement
The authors have no conflicts of interest to declare. Ritchie Centre, Hudson Institute of Medical Research, 27-31 Wright St, Clayton, Melbourne, Australia. Disclaimers: None.
Figures
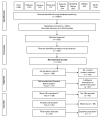
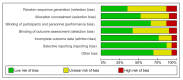
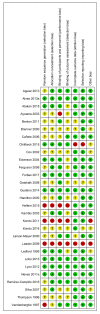

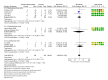


References
-
- Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992;281:21–40. doi: 10.1042/bj2810021. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

