Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update
- PMID: 32549331
- PMCID: PMC7345968
- DOI: 10.3390/md18060317
Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update
Abstract
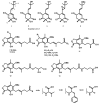
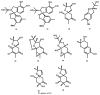
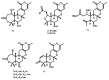
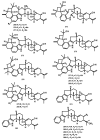
Meroterpenoids are a class of hybrid natural products, partially derived from a mixed terpenoid pathway. They possess remarkable structural features and relevant biological and pharmacological activities. Marine-derived fungi are a rich source of meroterpenoids featuring structural diversity varying from simple to complex molecular architectures. A combination of a structural variability and their myriad of bioactivities makes meroterpenoids an interesting class of naturally occurring compounds for chemical and pharmacological investigation. In this review, a comprehensive literature survey covering the period of 2009-2019, with 86 references, is presented focusing on chemistry and biological activities of various classes of meroterpenoids isolated from fungi obtained from different marine hosts and environments.
Keywords: anti-inflammatory; antibacterial; biological activities; cytotoxicity; marine-derived fungi; meroterpenoids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
























References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

