Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy
- PMID: 32549354
- PMCID: PMC7353010
- DOI: 10.3390/cancers12061584
Blood Flow Measurements Enable Optimization of Light Delivery for Personalized Photodynamic Therapy
Abstract
Fluence rate is an effector of photodynamic therapy (PDT) outcome. Lower light fluence rates can conserve tumor perfusion during some illumination protocols for PDT, but then treatment times are proportionally longer to deliver equivalent fluence. Likewise, higher fluence rates can shorten treatment time but may compromise treatment efficacy by inducing blood flow stasis during illumination. We developed blood-flow-informed PDT (BFI-PDT) to balance these effects. BFI-PDT uses real-time noninvasive monitoring of tumor blood flow to inform selection of irradiance, i.e., incident fluence rate, on the treated surface. BFI-PDT thus aims to conserve tumor perfusion during PDT while minimizing treatment time. Pre-clinical studies in murine tumors of radiation-induced fibrosarcoma (RIF) and a mesothelioma cell line (AB12) show that BFI-PDT preserves tumor blood flow during illumination better than standard PDT with continuous light delivery at high irradiance. Compared to standard high irradiance PDT, BFI-PDT maintains better tumor oxygenation during illumination and increases direct tumor cell kill in a manner consistent with known oxygen dependencies in PDT-mediated cytotoxicity. BFI-PDT promotes vascular shutdown after PDT, thereby depriving remaining tumor cells of oxygen and nutrients. Collectively, these benefits of BFI-PDT produce a significantly better therapeutic outcome than standard high irradiance PDT. Moreover, BFI-PDT requires ~40% less time on average to achieve outcomes that are modestly better than those with standard low irradiance treatment. This contribution introduces BFI-PDT as a platform for personalized light delivery in PDT, documents the design of a clinically-relevant instrument, and establishes the benefits of BFI-PDT with respect to treatment outcome and duration.
Keywords: Oxyphor; Photofrin®; blood flow monitoring; diffuse correlation spectroscopy; hemodynamic; light modulation; perfusion; phosphorescence quenching; photodynamic therapy; vascular response.
Conflict of interest statement
T.M.B., K.A.C., and T.C.Z. declare roles on the Advisory Board for Simphotek, Inc. The other authors declare no conflict of interest.
Figures
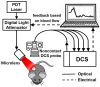
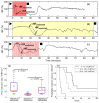
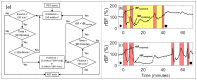
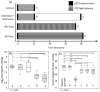

References
-
- Thong P., Lee K., Toh H.J., Dong J., Tee C.S., Low K.P., Chang P.H., Bhuvaneswari R., Tan N.C., Soo K.C. Early assessment of tumor response to photodynamic therapy using combined diffuse optical and diffuse correlation spectroscopy to predict treatment outcome. Oncotarget. 2017;8:19902–19913. doi: 10.18632/oncotarget.15720. - DOI - PMC - PubMed
-
- Fingar V.H., Wieman T.J., Wiehle S.A., Cerrito P.B. The role of microvascular damage in photodynamic therapy: The effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992;52:4914–4921. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

