Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways
- PMID: 32549377
- PMCID: PMC7352853
- DOI: 10.3390/ijms21124257
Lipid Mediators Regulate Pulmonary Fibrosis: Potential Mechanisms and Signaling Pathways
Abstract
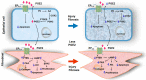
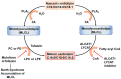
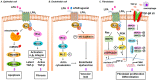
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease of unknown etiology characterized by distorted distal lung architecture, inflammation, and fibrosis. The molecular mechanisms involved in the pathophysiology of IPF are incompletely defined. Several lung cell types including alveolar epithelial cells, fibroblasts, monocyte-derived macrophages, and endothelial cells have been implicated in the development and progression of fibrosis. Regardless of the cell types involved, changes in gene expression, disrupted glycolysis, and mitochondrial oxidation, dysregulated protein folding, and altered phospholipid and sphingolipid metabolism result in activation of myofibroblast, deposition of extracellular matrix proteins, remodeling of lung architecture and fibrosis. Lipid mediators derived from phospholipids, sphingolipids, and polyunsaturated fatty acids play an important role in the pathogenesis of pulmonary fibrosis and have been described to exhibit pro- and anti-fibrotic effects in IPF and in preclinical animal models of lung fibrosis. This review describes the current understanding of the role and signaling pathways of prostanoids, lysophospholipids, and sphingolipids and their metabolizing enzymes in the development of lung fibrosis. Further, several of the lipid mediators and enzymes involved in their metabolism are therapeutic targets for drug development to treat IPF.
Keywords: G-protein coupled receptors; autotaxin; lipid mediators; lysocardiolipin acyltransferase; lysophosphatidic acid; oxidized phospholipids; phospholipase D; prostaglandins; pulmonary fibrosis; sphingolipids; sphingosine kinase 1; sphingosine-1-phosphate.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish this review article.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

