Molecular mechanosensors in osteocytes
- PMID: 32550039
- PMCID: PMC7280204
- DOI: 10.1038/s41413-020-0099-y
Molecular mechanosensors in osteocytes
Abstract
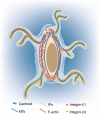
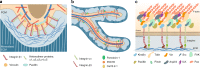
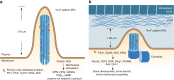
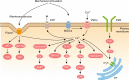
Osteocytes, the most abundant and long-lived cells in bone, are the master regulators of bone remodeling. In addition to their functions in endocrine regulation and calcium and phosphate metabolism, osteocytes are the major responsive cells in force adaptation due to mechanical stimulation. Mechanically induced bone formation and adaptation, disuse-induced bone loss and skeletal fragility are mediated by osteocytes, which sense local mechanical cues and respond to these cues in both direct and indirect ways. The mechanotransduction process in osteocytes is a complex but exquisite regulatory process between cells and their environment, between neighboring cells, and between different functional mechanosensors in individual cells. Over the past two decades, great efforts have focused on finding various mechanosensors in osteocytes that transmit extracellular mechanical signals into osteocytes and regulate responsive gene expression. The osteocyte cytoskeleton, dendritic processes, Integrin-based focal adhesions, connexin-based intercellular junctions, primary cilium, ion channels, and extracellular matrix are the major mechanosensors in osteocytes reported so far with evidence from both in vitro and in vitro studies. This review aims to give a systematic introduction to osteocyte mechanobiology, provide details of osteocyte mechanosensors, and discuss the roles of osteocyte mechanosensitive signaling pathways in the regulation of bone homeostasis.
Keywords: Bone quality and biomechanics; Osteoporosis.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

