Ultrashort echo time (UTE) magnetic resonance imaging of myelin: technical developments and challenges
- PMID: 32550129
- PMCID: PMC7276362
- DOI: 10.21037/qims-20-541
Ultrashort echo time (UTE) magnetic resonance imaging of myelin: technical developments and challenges
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-541). JD serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
Figures
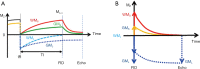


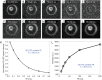






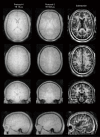


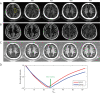
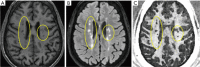
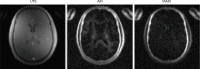
References
-
- van der Knaap MS, Valk J. Magnetic Resonance of Myelination and Myelin Disorders. Berlin: Springer, 2005;1-19.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
