Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic
- PMID: 32550266
- PMCID: PMC7280290
- DOI: 10.1038/s41523-020-0168-9
Evidence-based guidelines for managing patients with primary ER+ HER2- breast cancer deferred from surgery due to the COVID-19 pandemic
Abstract
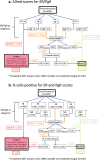
Many patients with ER+ HER2- primary breast cancer are being deferred from surgery to neoadjuvant endocrine therapy (NeoET) during the COVID-19 pandemic. We have collated data from multiple international trials of presurgical endocrine therapy in order to provide guidance on the identification of patients who may have insufficiently endocrine-sensitive tumors and should be prioritised for early surgery or neoadjuvant chemotherapy rather than NeoET during or in the aftermath of the COVID-19 pandemic for safety or when surgical activity needs to be prioritized. For postmenopausal patients, our data provide strong support for the use of ER and PgR status at diagnosis for triaging of patients into three groups in which (taking into account clinical factors): (i) NeoET is likely to be inappropriate (Allred ER <6 or ER 6 and PgR <6) (ii) a biopsy for Ki67 analysis (on-treatment Ki67) could be considered after 2-4 weeks of NeoET (a: ER 7 or 8 and PgR <6 or b: ER 6 or 7 and PgR ≥6) or (iii) NeoET is an acceptable course of action (ER 8 and PgR ≥6). Cut-offs for percentage of cells positive are also given. For group (ii), a high early on-treatment level of Ki67 (>10%) indicates a higher priority for early surgery. Too few data were available for premenopausal patients to provide a similar treatment algorithm. These guidelines should be helpful for managing patients with early ER+ HER2- breast cancer during and in the aftermath of the COVID-19 crisis.
Keywords: Breast cancer.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsM.D. is on advisory board in Lilly, H3Biomedicine, AbbVie, Orion and consultancy: Radius; Honoraria for lectures: Nanostring, Myriad. M.J.E. reports ownership and income from Veracyte/Nanostring for Royalties for Prosigna and ad hoc consulting fees from Novartis, Pfizer, AstraZenica, GI therapeutics, Foundation Medicine, Sermonix, Abbvie. O.G. has Lecture/consultancy fees from Celgene, Roche, Genomic Health, Amgen, Pfizer, Novartis, Lilly, Nanostring, Eisai, MSD; assistance with travel costs: Celgene, Roche, Daiichi Sankyo; S.K. reports personal fees from Roche/Genentech, Genomic Health, Novartis, Amgen, Celgene, Daiichi Sankyo, AstraZeneca, Somatex, MSD, Pfizer, Puma Biotechnology, PFM Medical, Lilly, Sonoscape. C.X.M. reports research funding from Pfizer, consulting fees from AstraZeneca, Novartis, Eli Lilly, Seattle Genetics, and Athenex Oncology. N.H. reports personal fees (lectures / consulting) from Agendia, Amgen, Astra Zeneca, BMS, Celgene, Daiichi-Sankyo, Genomic Health, Lilly, MSD, Novartis, Odonate, Pierre Fabre, Pfizer, Roche, Sandoz/Hexal, Seattle Genetics. The remaining authors declare no conflict of interests.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

