An oligoclonal combination of human monoclonal antibodies able to neutralize tetanus toxin in vivo
- PMID: 32550563
- PMCID: PMC7285915
- DOI: 10.1016/j.toxcx.2019.100006
An oligoclonal combination of human monoclonal antibodies able to neutralize tetanus toxin in vivo
Abstract
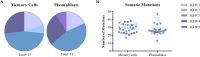
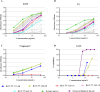
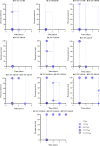
The use of antibody-based therapy to treat a variety of diseases and conditions is well documented. The use of antibodies as an antidote to treat tetanus infections was one of the first examples of immunotherapy and remains the standard of care for cases involving potential infections. Plasma-derived immunoglobulins obtained from human or horse pose risks of infection from undetectable emergent viruses or may cause anaphylaxis. Further, there is a lack of consistency between lots. In the search for new formulations, we obtained a series of clonally related human monoclonal antibodies (mAbs) derived from B cells sorted from donors that presented anti-tetanus neutralizing titers. Donors were revaccinated prior to blood collection. Different strategies were used for single-cell sorting, since it was challenging to identify cells at a very low frequency: memory B cell sorting using fluorescent-labeled tetanus toxoid and toxin as baits, and plasmablast sorting done shortly after revaccination. Screening of the recombinant mAbs with the whole tetanus toxin allowed us to select candidates with therapeutic potential, since mAbs to different domains can contribute additively to the neutralizing effect. Because of selective binding to different domains, we tested mAbs individually, or in mixtures of two or three, in the neutralizing in vivo assay specified by Pharmacopeia for the determination of polyclonal hyperimmune sera potency. An oligoclonal mixture of three human mAbs completely neutralized the toxin injected in the animals, signaling an important step for clinical mAb development.
Keywords: Epitope mapping; Ganglioside; Neuron receptor; Neutralizing antibody; Peptide array; Tetanus toxin.
© 2019 The Author(s).
Figures



References
LinkOut - more resources
Full Text Sources

