Comparative characterization of Viperidae snake venoms from Perú reveals two compositional patterns of phospholipase A2 expression
- PMID: 32550596
- PMCID: PMC7285926
- DOI: 10.1016/j.toxcx.2020.100044
Comparative characterization of Viperidae snake venoms from Perú reveals two compositional patterns of phospholipase A2 expression
Abstract
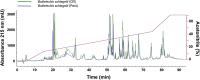
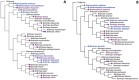
Snake species within the Bothrops complex (sensu lato) are of medical relevance in Latin America, but knowledge on their venom characteristics is limited, or even unavailable, for some taxa. Perú harbors 17 species of pit vipers, within the genera Bothrops, Bothriechis, Bothrocophias, Porthidium, Crotalus, and Lachesis. This study compared the venoms of twelve species, through chromatographic and electrophoretic profiles, as well as proteolytic and phospholipase A2 (PLA2) activities. Also, proteomic profiles were analyzed for nine of the venoms using a shotgun approach. Results unveiled conspicuous differences in the expression of venom PLA2s among species, six of them presenting scarce levels as judged by RP-HPLC profiles. Since most species within the bothropoid lineage possess venoms with high to intermediate abundances of this protein family, our findings suggest the existence of a phenotypic duality in the expression of venom PLA2s within the Bothrops (sensu lato) complex. Bothrops barnetti and Bothrocophias andianus venoms, very scarce in PLA2s, were shown to lack significant myotoxic activity, highlighting that the observed variability in PLA2 expression bears toxicological correlations with effects attributed to these proteins. Finally, an attempt to identify phylogenetic relationships of bothropoid species from Perú presenting low- or high-PLA2 venom phenotypes showed an interspersed pattern, thus precluding a simple phylogenetic interpretation of this venom compositional dichotomy.
Keywords: Bothropoid; Dichotomy; Phenotype; Phospholipase A2; Snake venom; Viperidae.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests related to this work.
Figures







Similar articles
-
Crystal structure of a phospholipase A2 from Bothrops asper venom: Insights into a new putative "myotoxic cluster".Biochimie. 2017 Feb;133:95-102. doi: 10.1016/j.biochi.2016.12.015. Epub 2016 Dec 27. Biochimie. 2017. PMID: 28034717
-
Antivenomics and in vivo preclinical efficacy of six Latin American antivenoms towards south-western Colombian Bothrops asper lineage venoms.PLoS Negl Trop Dis. 2021 Feb 1;15(2):e0009073. doi: 10.1371/journal.pntd.0009073. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33524033 Free PMC article.
-
New insights into the phylogeographic distribution of the 3FTx/PLA2 venom dichotomy across genus Micrurus in South America.J Proteomics. 2019 May 30;200:90-101. doi: 10.1016/j.jprot.2019.03.014. Epub 2019 Apr 1. J Proteomics. 2019. PMID: 30946991
-
Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics.J Proteomics. 2014 Jun 13;105:323-39. doi: 10.1016/j.jprot.2014.02.020. Epub 2014 Feb 24. J Proteomics. 2014. PMID: 24576642 Review.
-
South American snake venoms with abundant neurotoxic components. Composition and toxicological properties. A literature review.Acta Trop. 2021 Dec;224:106119. doi: 10.1016/j.actatropica.2021.106119. Epub 2021 Sep 2. Acta Trop. 2021. PMID: 34481791 Review.
Cited by
-
Venom Composition of Neglected Bothropoid Snakes from the Amazon Rainforest: Ecological and Toxinological Implications.Toxins (Basel). 2024 Feb 4;16(2):83. doi: 10.3390/toxins16020083. Toxins (Basel). 2024. PMID: 38393161 Free PMC article.
-
Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications.Toxins (Basel). 2025 Mar 14;17(3):136. doi: 10.3390/toxins17030136. Toxins (Basel). 2025. PMID: 40137909 Free PMC article.
-
From birth to adulthood: An analysis of the Brazilian lancehead (Bothrops moojeni) venom at different life stages.PLoS One. 2021 Jun 10;16(6):e0253050. doi: 10.1371/journal.pone.0253050. eCollection 2021. PLoS One. 2021. PMID: 34111213 Free PMC article.
-
Elucidating the Venom Diversity in Sri Lankan Spectacled Cobra (Naja naja) through De Novo Venom Gland Transcriptomics, Venom Proteomics and Toxicity Neutralization.Toxins (Basel). 2021 Aug 10;13(8):558. doi: 10.3390/toxins13080558. Toxins (Basel). 2021. PMID: 34437429 Free PMC article.
-
Biochemical and hemostatic description of a thrombin-like enzyme TLBro from Bothrops roedingeri snake venom.Front Chem. 2023 Nov 30;11:1217329. doi: 10.3389/fchem.2023.1217329. eCollection 2023. Front Chem. 2023. PMID: 38099189 Free PMC article.
References
-
- Ainsworth S., Petras D., Engmark M., Süssmuth R.D., Whiteley G., Albulescu L.O., Kazandjian T.D., Wagstaff S.C., Rowley P., Wüster W., Dorrestein P.C., Arias A.S., Gutiérrez J.M., Harrison R.A., Casewell N.R., Calvete J.J. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J. Proteomics. 2018;172:173–189. - PubMed
-
- Alape-Girón A., Sanz L., Escolano J., Flores-Díaz M., Madrigal M., Sasa M., Calvete J.J. Snake venomics of the lancehead pitviper Bothrops asper: geographic, individual, and ontogenetic variations. J. Proteome Res. 2008;7:3556–3571. - PubMed
-
- Alencar L.R.V., Quental T.B., Grazziotin F.G., Alfaro M.L., Martins M., Venzon M., Zaher H. Diversification in vipers: phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 2016;105:50–62. - PubMed

