Errors within the total laboratory testing process, from test selection to medical decision-making - A review of causes, consequences, surveillance and solutions
- PMID: 32550813
- PMCID: PMC7271754
- DOI: 10.11613/BM.2020.020502
Errors within the total laboratory testing process, from test selection to medical decision-making - A review of causes, consequences, surveillance and solutions
Abstract
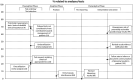
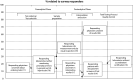
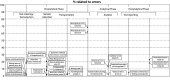
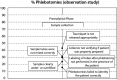
Laboratory analyses are crucial for diagnosis, follow-up and treatment decisions. Since mistakes in every step of the total testing process may potentially affect patient safety, a broad knowledge and systematic assessment of laboratory errors is essential for future improvement. In this review, we aim to discuss the types and frequencies of potential errors in the total testing process, quality management options, as well as tentative solutions for improvement. Unlike most currently available reviews on this topic, we also include errors in test-selection, reporting and interpretation/action of test results. We believe that laboratory specialists will need to refocus on many process steps belonging to the extra-analytical phases, intensifying collaborations with clinicians and supporting test selection and interpretation. This would hopefully lead to substantial improvements in these activities, but may also bring more value to the role of laboratory specialists within the health care setting.
Keywords: extra-analytical phase; laboratory medicine; patient safety; quality indicators; total testing process.
Croatian Society of Medical Biochemistry and Laboratory Medicine.
Conflict of interest statement
Potential conflict of interest: None declared.
Figures


References
-
- Bucurescu S. Pre-analytical Laboratory Error in a Stroke Patient due to Blood Collection from another Stroke Patient: A Case Report. J Neurol Neurophysiol. 2013;4:178. 10.4172/2155-9562.1000178 - DOI

