Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation
- PMID: 32550919
- PMCID: PMC7295059
- DOI: 10.7150/thno.45359
Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation
Abstract
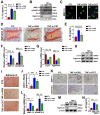
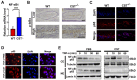
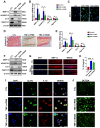
Background: Intervertebral disc (IVD) degeneration is a common degenerative disease that can lead to collapse or herniation of the nucleus pulposus (NP) and result in radiculopathy in patients. Methods: NP tissue and cells were isolated from patients and mice, and the expression profile of cortistatin (CST) was analysed. In addition, ageing of the NP was compared between 6-month-old WT and CST-knockout (CST-/-) mice. Furthermore, NP tissues and cells were cultured to validate the role of CST in TNF-α-induced IVD degeneration. Moreover, in vitro and in vivo experiments were performed to identify the potential role of CST in mitochondrial dysfunction, mitochondrial ROS generation and activation of the NLRP3 inflammasome during IVD degeneration. In addition, NF-κB signalling pathway activity was tested in NP tissues and cells from CST-/- mice. Results: The expression of CST in NP cells was diminished in the ageing- and TNF-α-induced IVD degeneration process. In addition, compared with WT mice, aged CST-/- mice displayed accelerated metabolic imbalance and enhanced apoptosis, and these mice showed a disorganized NP tissue structure. Moreover, TNF-α-mediated catabolism and apoptosis were alleviated by exogenous CST treatment. Furthermore, CST inhibited mitochondrial dysfunction in NP cells through IVD degeneration and suppressed activation of the NLRP3 inflammasome. In vitro and ex vivo experiments indicated that increased NF-κB pathway activity might have been associated with the IVD degeneration observed in CST-/- mice. Conclusion: This study suggests the role of CST in mitochondrial ROS and activation of the NLRP3 inflammasome in IVD degeneration, which might shed light on therapeutic targets for IVD degeneration.
Keywords: Intervertebral disc degeneration; NF-κB signalling pathway; NLRP3; cortistatin; mitochondrial ROS.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Desmoulin GT, Pradhan V, Milner TE. Mechanical Aspects of Intervertebral Disc Injury and Implications on Biomechanics. Spine. 2019;20:E457–e64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

