Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase
- PMID: 32551007
- PMCID: PMC7294724
- DOI: 10.1021/acsmedchemlett.0c00102
Computer-Aided Fragment Growing Strategies to Design Dual Inhibitors of Soluble Epoxide Hydrolase and LTA4 Hydrolase
Abstract
Multitarget ligands are interesting candidates for drug discovery and development due to improved safety and efficacy. However, rational design and optimization of multitarget ligands is tedious because affinity optimization for two or more targets has to be performed simultaneously. In this study, we demonstrate that, given a molecular fragment, which binds to two targets of interest, computer-aided fragment growing can be applied to optimize compound potency, relying on either ligand- or structure-derived information. This methodology is applied to the design of dual inhibitors of soluble epoxide hydrolase and leukotriene A4 hydrolase.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
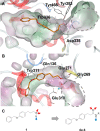
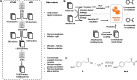
References
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

