Analgesic efficacy of nefopam for cancer pain: a randomized controlled study
- PMID: 32551097
- PMCID: PMC7276938
- DOI: 10.12688/f1000research.23455.1
Analgesic efficacy of nefopam for cancer pain: a randomized controlled study
Abstract
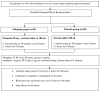
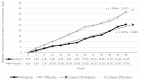
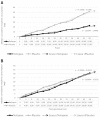
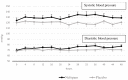
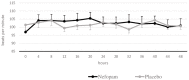
Background: Nefopam is a non-opioid, non-steroidal, central acting drug used effectively for postoperative pain. The efficacy of nefopam for cancer pain remains unclear. We aimed to evaluate the analgesic efficacy of nefopam for cancer pain in a randomized controlled trial. Methods: Patients with moderate to severe cancer pain (n=40) were randomly divided into two groups. The nefopam group (n=20) received three 20 mg doses of nefopam every 8 hours. The placebo group (n=20) received normal saline. Intravenous patient-controlled analgesia with morphine was given for breakthrough pain for 48 hours. The primary outcome was significant pain reduction. Secondary outcomes were morphine consumption over 48 hours and incidence of side effects. Results: The nefopam group showed pain reduction at 12 hours (65% of patients), 24 hours (80%), 36 hours (85%), and 48 hours (65%). The placebo group showed pain reduction at 12 hours (70%), 24 hours (75%), 36 hours (80%), and 48 hours (60%). However, there were no statistically significant differences between the groups (p>0.05). The median dosage of morphine consumption in 48 hours was lower in the nefopam group (25.5 mg) compared with the placebo group (37 mg), but this was not statistically significant (p=0.499). There were no statistically significant differences in blood pressure and heart rate between the groups. Side effects in both groups were comparable. Conclusions: At dosage of 60 mg in 24 hours, nefopam did not provide significant pain reduction in moderate to severe cancer pain patients. However, there was a trend of reduced opioid consumption. Further studies with larger sample sizes, longer duration, or higher doses of nefopam are warranted. Registration: Thai Clinical Trail Registry (TCTR) ID TCTR20181016001; registered on 12 October 2018.
Keywords: analgesia; cancer; efficacy; morphine consumption; nefopam; pain; pain reduction; patient controlled analgesia; side effect.
Copyright: © 2020 Pasutharnchat K et al.
Conflict of interest statement
No competing interests were disclosed.
Figures

References
-
- World Health Organization: WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents.2018. Reference Source - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

