Electrochemical nitrite sensing for urine nitrification
- PMID: 32551436
- PMCID: PMC7287277
- DOI: 10.1016/j.wroa.2020.100055
Electrochemical nitrite sensing for urine nitrification
Abstract
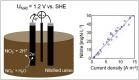
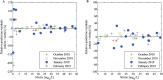
Sensing nitrite in-situ in wastewater treatment processes could greatly simplify process control, especially during treatment of high-strength nitrogen wastewaters such as digester supernatant or, as in our case, urine. The two technologies available today, i.e. an on-line nitrite analyzer and a spectrophotometric sensor, have strong limitations such as sample preparation, cost of ownership and strong interferences. A promising alternative is the amperometric measurement of nitrite, which we assessed in this study. We investigated the sensor in a urine nitrification reactor and in ex-situ experiments. Based on theoretical calculations as well as a practical approach, we determined that the critical nitrite concentrations for nitrite oxidizing bacteria lie between 12 and 30 mgN/L at pH 6 to 6.8. Consequently, we decided that the sensor should be able to reliably measure concentrations up to 50 mgN/L, which is about double the value of the critical nitrite concentration. We found that the influences of various ambient conditions, such as temperature, pH, electric conductivity and aeration rate, in the ranges expected in urine nitrification systems, are negligible. For low nitrite concentrations, as expected in municipal wastewater treatment, the tested amperometric nitrite sensor was not sufficiently sensitive. Nevertheless, the sensor delivered reliable measurements for nitrite concentrations of 5-50 mgN/L or higher. This means that the amperometric nitrite sensor allows detection of critical nitrite concentrations without difficulty in high-strength nitrogen conversion processes, such as the nitrification of human urine.
Keywords: Amperometric sensor; Continuous measurement; Critical nitrite concentration; Electrochemical measurement; In-situ measurement; Nitrite measurement.
© 2020 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kai M. Udert is co-owner of the Eawag spin-off Vuna Ltd, which has a license for electrochemical nitrite removal and control. Peter Schrems produced the small nitrite sensor.
Figures












References
-
- Ahmad Z. Elsevier Butterworth-Heinemann; Amsterdam: 2006. Principles of Corrosion Engineering and Corrosion Control.
-
- Anthonisen A.C., Loehr R.C., Prakasam T.B.S., Srinath E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Health.com. 1976;48(5):835–852. - PubMed
-
- Deshpande A.P., Patwardhan S.C., Narasimhan S.S. Intelligent state estimation for fault tolerant nonlinear predictive control. J. Process Contr. 2009;19(2):187–204. doi: 10.1016/j.jprocont.2008.04.006. - DOI
-
- Ellram L.M. Total cost of ownership. Int. J. Phys. Distrib. Logist. Manag. 1995
LinkOut - more resources
Full Text Sources

