Gallium(III)-Salophen as a Dual Inhibitor of Pseudomonas aeruginosa Heme Sensing and Iron Acquisition
- PMID: 32551497
- PMCID: PMC7456513
- DOI: 10.1021/acsinfecdis.0c00138
Gallium(III)-Salophen as a Dual Inhibitor of Pseudomonas aeruginosa Heme Sensing and Iron Acquisition
Abstract
Pseudomonas aeruginosa is an opportunistic bacterium that causes life-threatening infections in immunocompromised patients. In infection, it uses heme as a primary iron source and senses the availability of exogenous heme through the heme assimilation system (Has), an extra cytoplasmic function σ-factor system. A secreted hemophore HasAp scavenges heme and, upon interaction with the outer-membrane receptor HasR, activates a signaling cascade, which in turn creates a positive feedback loop critical for sensing and adaptation within the host. The ability to sense and respond to heme as an iron source contributes to virulence. Consequently, the inhibition of this system will lead to a disruption in iron homeostasis, decreasing virulence. We have identified a salophen scaffold that successfully inhibits the activation of the Has signaling system while simultaneously targeting iron uptake via xenosiderophore receptors. We propose this dual mechanism wherein free Ga3+-salophen reduces growth through uptake and iron mimicry. A dual mechanism targeting extracellular heme signaling and uptake together with Ga3+-induced toxicity following active Ga3+salophen uptake provides a significant therapeutic advantage while reducing the propensity to develop resistance.
Keywords: Pseudomonas aeruginosa; antimicrobials; heme sensing; heme uptake; metallotherapeutics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

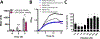


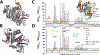
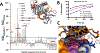
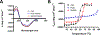
Similar articles
-
Contributions of the heme coordinating ligands of the Pseudomonas aeruginosa outer membrane receptor HasR to extracellular heme sensing and transport.J Biol Chem. 2020 Jul 24;295(30):10456-10467. doi: 10.1074/jbc.RA120.014081. Epub 2020 Jun 10. J Biol Chem. 2020. PMID: 32522817 Free PMC article.
-
Differential contributions of the outer membrane receptors PhuR and HasR to heme acquisition in Pseudomonas aeruginosa.J Biol Chem. 2015 Mar 20;290(12):7756-66. doi: 10.1074/jbc.M114.633495. Epub 2015 Jan 22. J Biol Chem. 2015. PMID: 25616666 Free PMC article.
-
Post-transcriptional regulation of the Pseudomonas aeruginosa heme assimilation system (Has) fine-tunes extracellular heme sensing.J Biol Chem. 2019 Feb 22;294(8):2771-2785. doi: 10.1074/jbc.RA118.006185. Epub 2018 Dec 28. J Biol Chem. 2019. PMID: 30593511 Free PMC article.
-
Heme acquisition by hemophores.Biometals. 2007 Jun;20(3-4):603-13. doi: 10.1007/s10534-006-9050-y. Epub 2007 Feb 1. Biometals. 2007. PMID: 17268821 Review.
-
Targeting iron uptake to control Pseudomonas aeruginosa infections in cystic fibrosis.Eur Respir J. 2013 Dec;42(6):1723-36. doi: 10.1183/09031936.00124012. Epub 2012 Nov 8. Eur Respir J. 2013. PMID: 23143541 Review.
Cited by
-
The role of bacterial metabolism in antimicrobial resistance.Nat Rev Microbiol. 2025 Jul;23(7):439-454. doi: 10.1038/s41579-025-01155-0. Epub 2025 Feb 20. Nat Rev Microbiol. 2025. PMID: 39979446 Free PMC article. Review.
-
Advantageous Reactivity of Unstable Metal Complexes: Potential Applications of Metal-Based Anticancer Drugs for Intratumoral Injections.Pharmaceutics. 2022 Apr 4;14(4):790. doi: 10.3390/pharmaceutics14040790. Pharmaceutics. 2022. PMID: 35456624 Free PMC article. Review.
-
Mechanisms of iron homeostasis in Pseudomonas aeruginosa and emerging therapeutics directed to disrupt this vital process.Microb Biotechnol. 2023 Jul;16(7):1475-1491. doi: 10.1111/1751-7915.14241. Epub 2023 Mar 1. Microb Biotechnol. 2023. PMID: 36857468 Free PMC article. Review.
-
Iron Homeostasis in Pseudomonas aeruginosa: Targeting Iron Acquisition and Storage as an Antimicrobial Strategy.Adv Exp Med Biol. 2022;1386:29-68. doi: 10.1007/978-3-031-08491-1_2. Adv Exp Med Biol. 2022. PMID: 36258068
-
Corrole-protein interactions in H-NOX and HasA.RSC Chem Biol. 2022 Mar 21;3(5):571-581. doi: 10.1039/d2cb00004k. eCollection 2022 May 11. RSC Chem Biol. 2022. PMID: 35656484 Free PMC article.
References
-
- CDC. (2019) in Antibiotic Resistance Threats in the United States, 2019; Center of Disease Control and Prevention, Atlanta, GA.
-
- Kerr KG, and Snelling AM (2009) Pseudomonas aeruginosa: A Formidable and Ever-Present Adversary. J. Hosp. Infect 73, 338–344. - PubMed
-
- Costerton JW (2001) Cystic Fibrosis Pathogenesis and the Role of Biofilms in Persistent Infection. Trends Microbiol 9, 50–52. - PubMed
-
- Burrows LL (2018) The Therapeutic Pipeline for Pseudomonas aeruginosa Infections. ACS Infect. Dis 4, 1041–1047. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

