Examining the Generality of Metal-Ligand Cooperativity Across a Series of First-Row Transition Metals: Capture, Bond Activation, and Stabilization
- PMID: 32551605
- PMCID: PMC7340558
- DOI: 10.1021/acs.inorgchem.0c01163
Examining the Generality of Metal-Ligand Cooperativity Across a Series of First-Row Transition Metals: Capture, Bond Activation, and Stabilization
Abstract
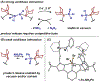
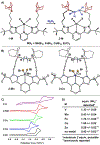
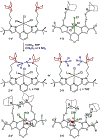
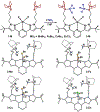
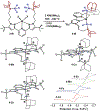
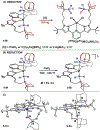
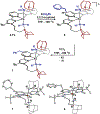
We outline the generality and requirements for cooperative N2H4 capture, N-N bond scission, and amido stabilization across a series of first-row transition metal complexes bearing a pyridine(dipyrazole) ligand. This ligand contains a pair of flexibly tethered trialkylborane Lewis acids that enable hydrazine capture and M-NH2 stabilization. While the Lewis acids are required to bind N2H4, the identity of the metal dictates whether N-N bond scission can occur. The redox properties of the M(II) bis(amidoborane) series of complexes were investigated and reveal that ligand-based events prevail; oxidation results in the generation of a transiently formed aminyl radical, while reduction occurs at the redox-active pyridine(dipyrazole) ligand.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
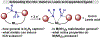
Similar articles
-
Through the Looking Glass: Using the Lens of [SNS]-Pincer Ligands to Examine First-Row Metal Bifunctional Catalysts.Acc Chem Res. 2023 Apr 4;56(7):798-809. doi: 10.1021/acs.accounts.2c00798. Epub 2023 Mar 15. Acc Chem Res. 2023. PMID: 36921212
-
A periodic walk: a series of first-row transition metal complexes with the pentadentate ligand PY5.Inorg Chem. 2002 Sep 9;41(18):4633-41. doi: 10.1021/ic025617r. Inorg Chem. 2002. PMID: 12206686
-
Additive Effects in Metal/Lewis Acid Cooperativity Assessed in a Tetrahedral Copper Hydrazine Complex Featuring an Appended Borane.Inorg Chem. 2024 Oct 7;63(40):18519-18523. doi: 10.1021/acs.inorgchem.4c02865. Epub 2024 Sep 17. Inorg Chem. 2024. PMID: 39287153
-
Metal Complexes of Redox Non-Innocent Ligand N,N'-Bis(3,5-di-tertbutyl-2-hydroxy-phenyl)-1,2-phenylenediamine.Molecules. 2024 Feb 29;29(5):1088. doi: 10.3390/molecules29051088. Molecules. 2024. PMID: 38474599 Free PMC article. Review.
-
Dinickel Active Sites Supported by Redox-Active Ligands.Acc Chem Res. 2021 Oct 5;54(19):3710-3719. doi: 10.1021/acs.accounts.1c00424. Epub 2021 Sep 26. Acc Chem Res. 2021. PMID: 34565142 Free PMC article. Review.
Cited by
-
Mobility of Lewis acids within the secondary coordination sphere: toward a model for cooperative substrate binding.Chem Commun (Camb). 2020 Nov 7;56(86):13105-13108. doi: 10.1039/d0cc05121g. Epub 2020 Oct 5. Chem Commun (Camb). 2020. PMID: 33016291 Free PMC article.
-
Appended Lewis Acids Enable Dioxygen Reactivity and Catalytic Oxidations with Ni(II).J Am Chem Soc. 2024 May 8;146(18):12375-12385. doi: 10.1021/jacs.3c12399. Epub 2024 Apr 25. J Am Chem Soc. 2024. PMID: 38661576 Free PMC article.
-
A Bidentate Ligand Featuring Ditopic Lewis Acids in the Second Sphere for Selective Substrate Capture and Activation.Angew Chem Int Ed Engl. 2023 Mar 20;62(13):e202218907. doi: 10.1002/anie.202218907. Epub 2023 Feb 20. Angew Chem Int Ed Engl. 2023. PMID: 36720708 Free PMC article.
References
-
- Smil V, Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. 1st ed.; MIT Press: Cambridge, MA: 2001.
-
- Arashiba K; Miyake Y; Nishibayashi Y, A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem 2010, 3, 120. - PubMed
-
- Yandulov DV; Schrock RR, Catalytic Reduction of Dinitrogen to Ammonia at a Single Molybdenum Center. Science 2003, 301, 76. - PubMed
-
- Laplaza CE; Cummins CC, Dinitrogen Cleavage by a Three-Coordinate Molybdenum(III) Complex. Science 1995, 268, 861. - PubMed
-
- Tanabe Y; Nishibayashi Y, Recent advances in nitrogen fixation upon vanadium complexes. Coord. Chem. Rev 2019, 381, 135–150.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

